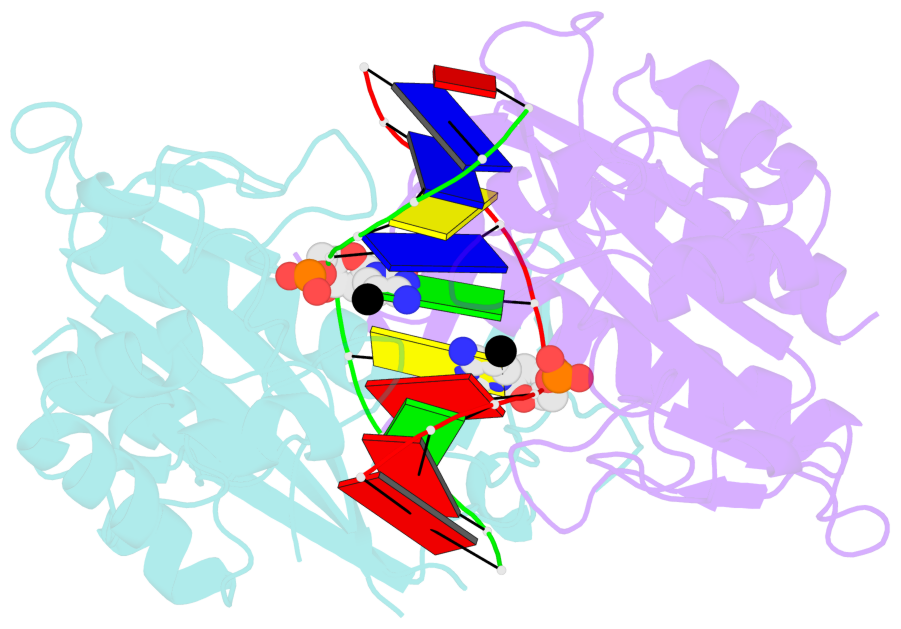
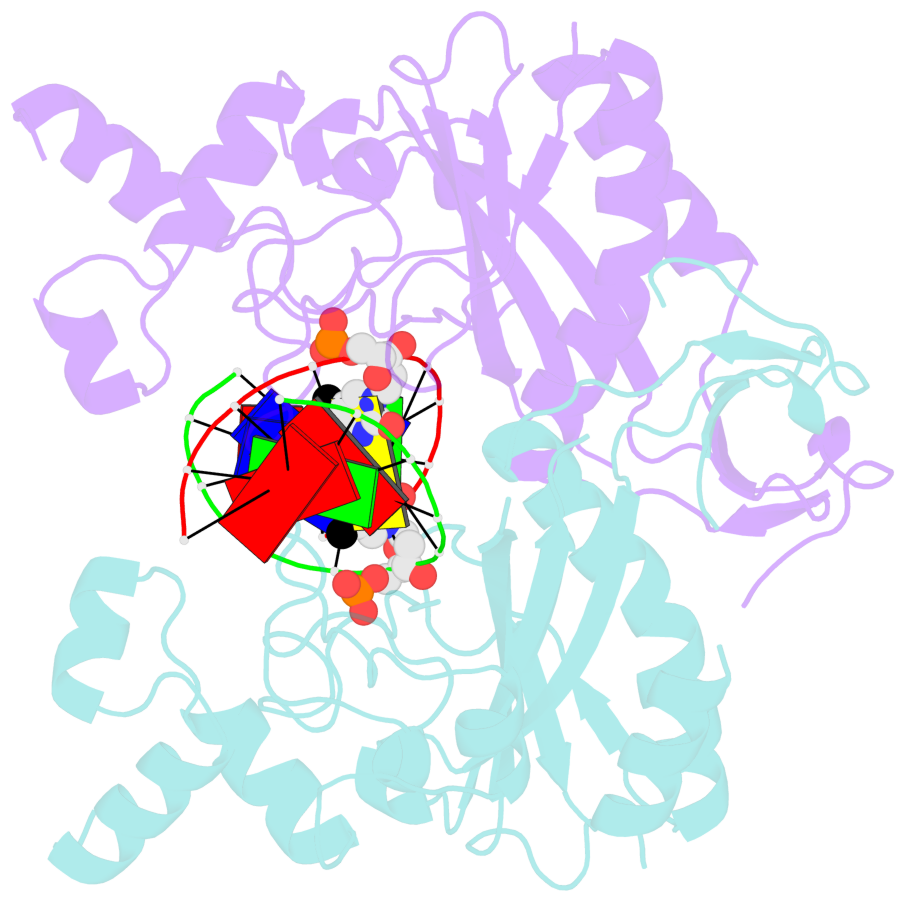
Summary information and primary citation
- PDB-id
- 1bsu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.0 Å)
- Summary
- Structural and energetic origins of indirect readout in site-specific DNA cleavage by a restriction endonuclease
- Reference
- Martin AM, Sam MD, Reich NO, Perona JJ (1999): "Structural and energetic origins of indirect readout in site-specific DNA cleavage by a restriction endonuclease." Nat.Struct.Biol., 6, 269-277. doi: 10.1038/6707.
- Abstract
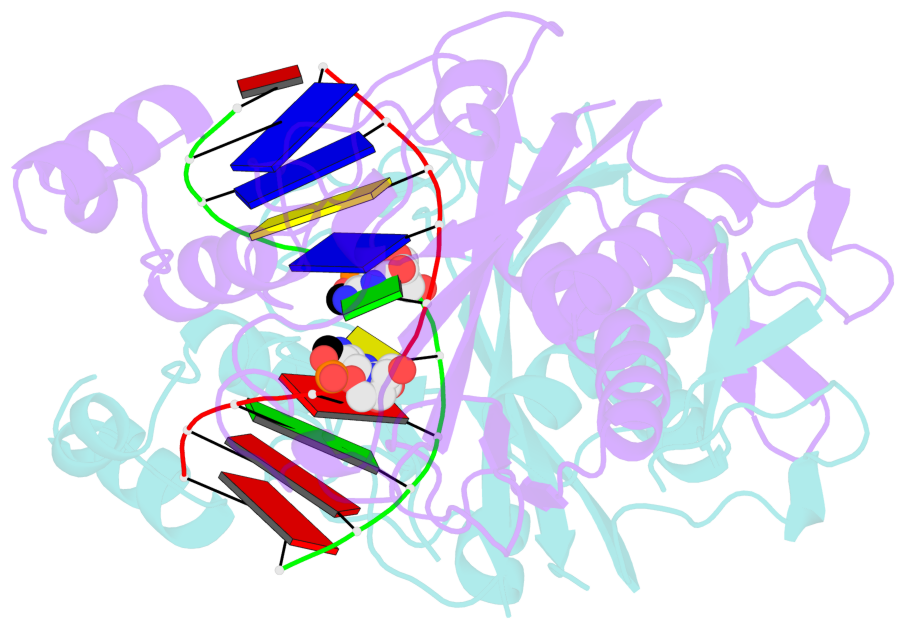
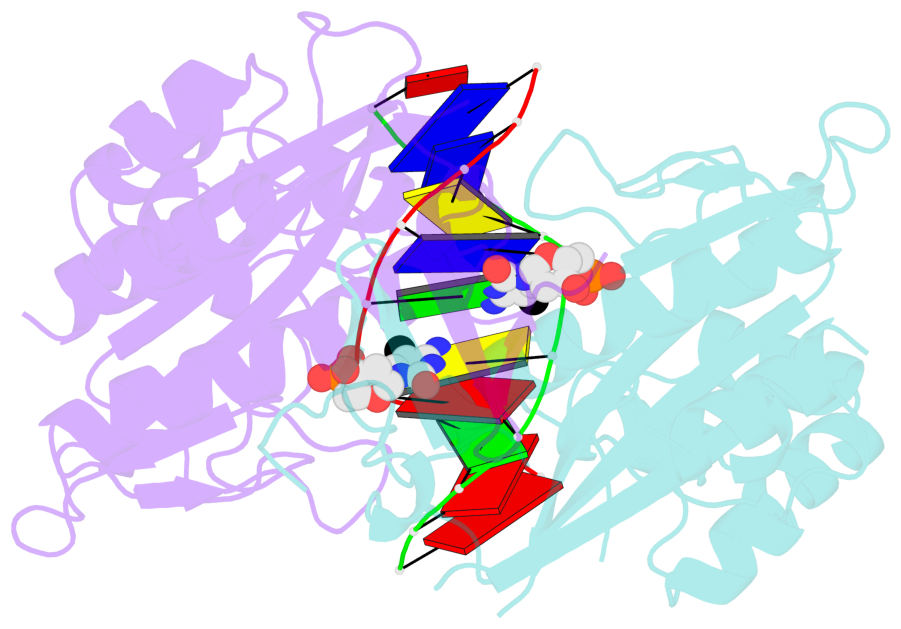
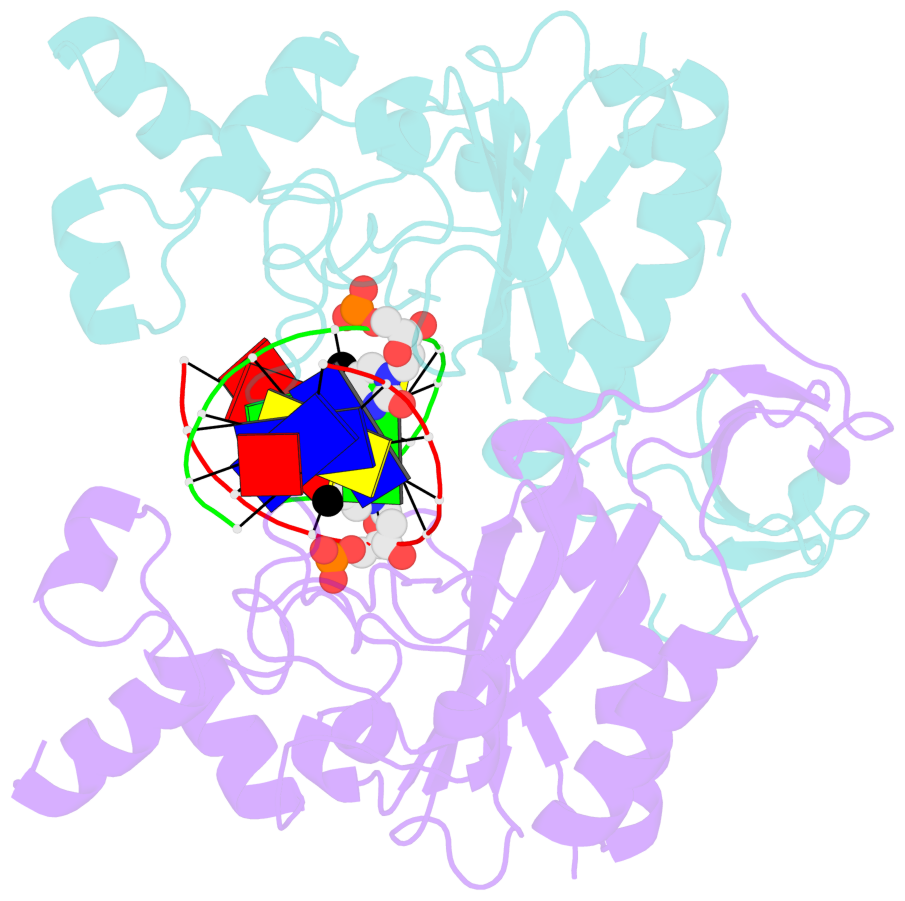
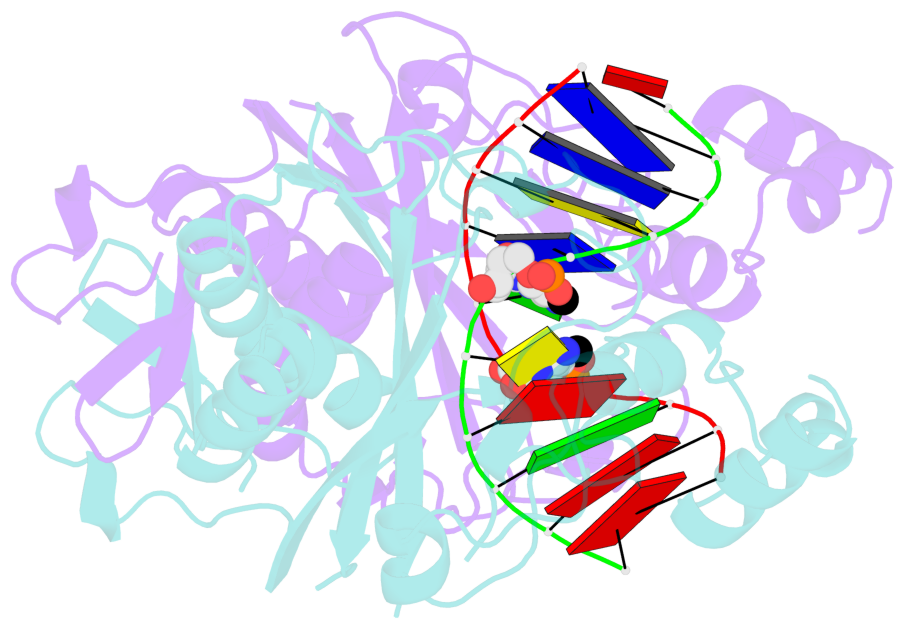
- Specific recognition by EcoRV endonuclease of its cognate, sharply bent GATATC site at the center TA step occurs solely via hydrophobic interaction with thymine methyl groups. Mechanistic kinetic analyses of base analog-substituted DNAs at this position reveal that direct readout provides 5 kcal mol(-1) toward specificity, with an additional 6-10 kcal mol(-1) arising from indirect readout. Crystal structures of several base analog complexes show that the major-groove hydrophobic contacts are crucial to forming required divalent metal-binding sites, and that indirect readout operates in part through the sequence-dependent free-energy cost of unstacking the center base-pair step of the DNA.
- The contacts include paired nucleotides (mostly a G in Watson-Crick G-C pairing), and
amino-acids within a 4.5-A distance cutoff to base atoms of 5mC.
- The structure is oriented in the base reference frame of 5mC, allowing for easy comparison
and direct superimposition between entries.
- The black sphere (•) denotes the 5-methyl carbon atom in 5mC.
No. 1 C.5CM906: other-contacts is-WC-paired is-in-duplex [+]:Acg/cgT |
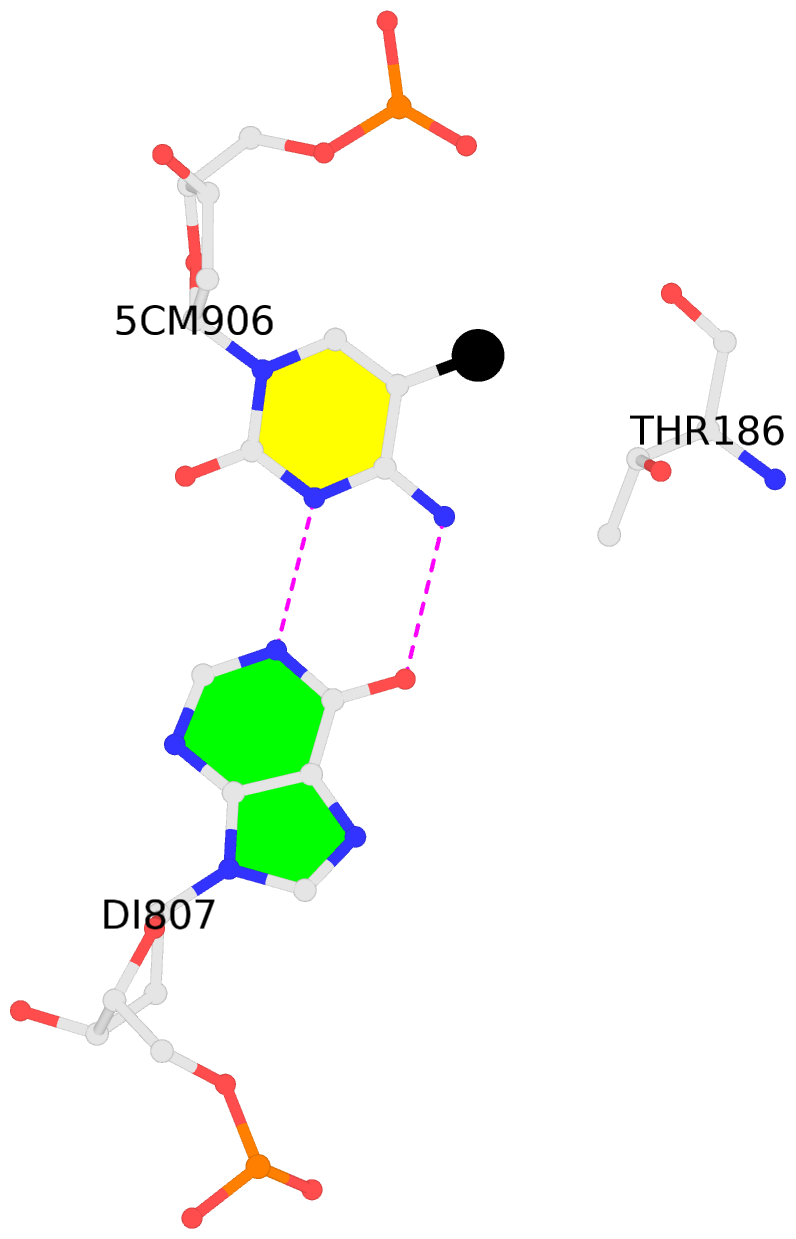 |
|
No. 2 D.5CM806: other-contacts is-WC-paired is-in-duplex [-]:cgT/Acg |
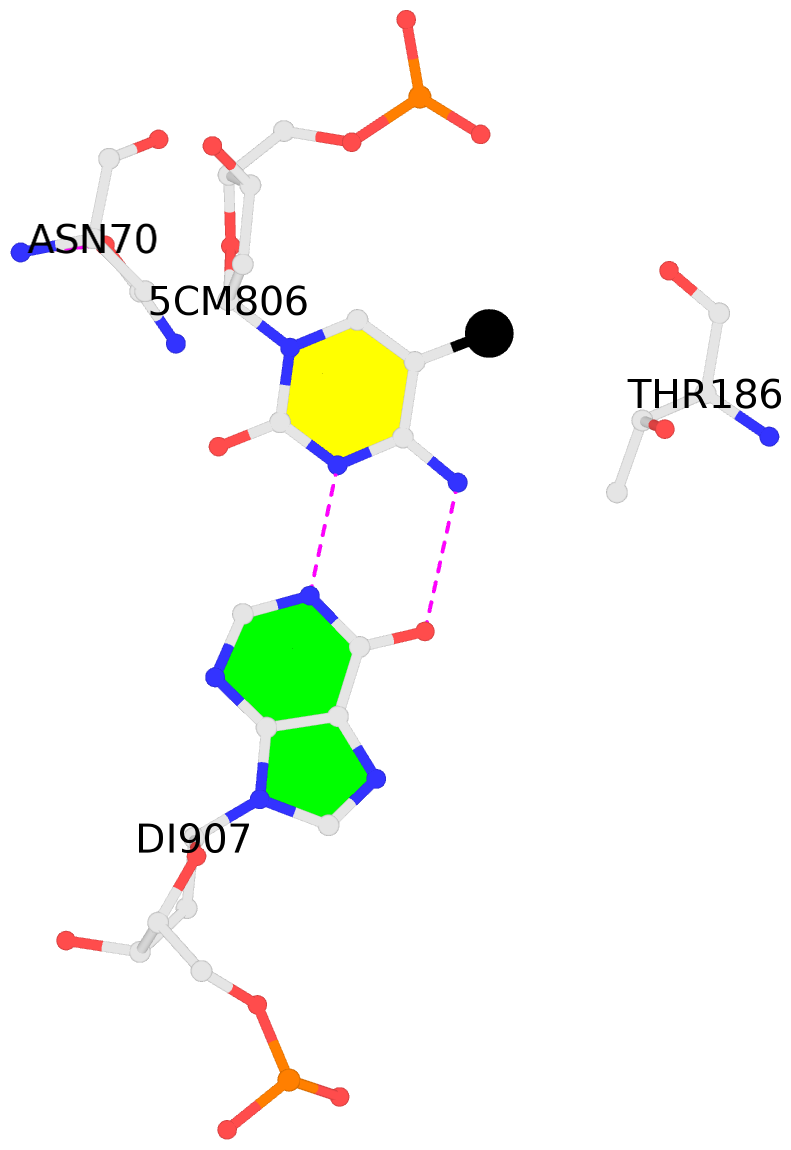 |
|