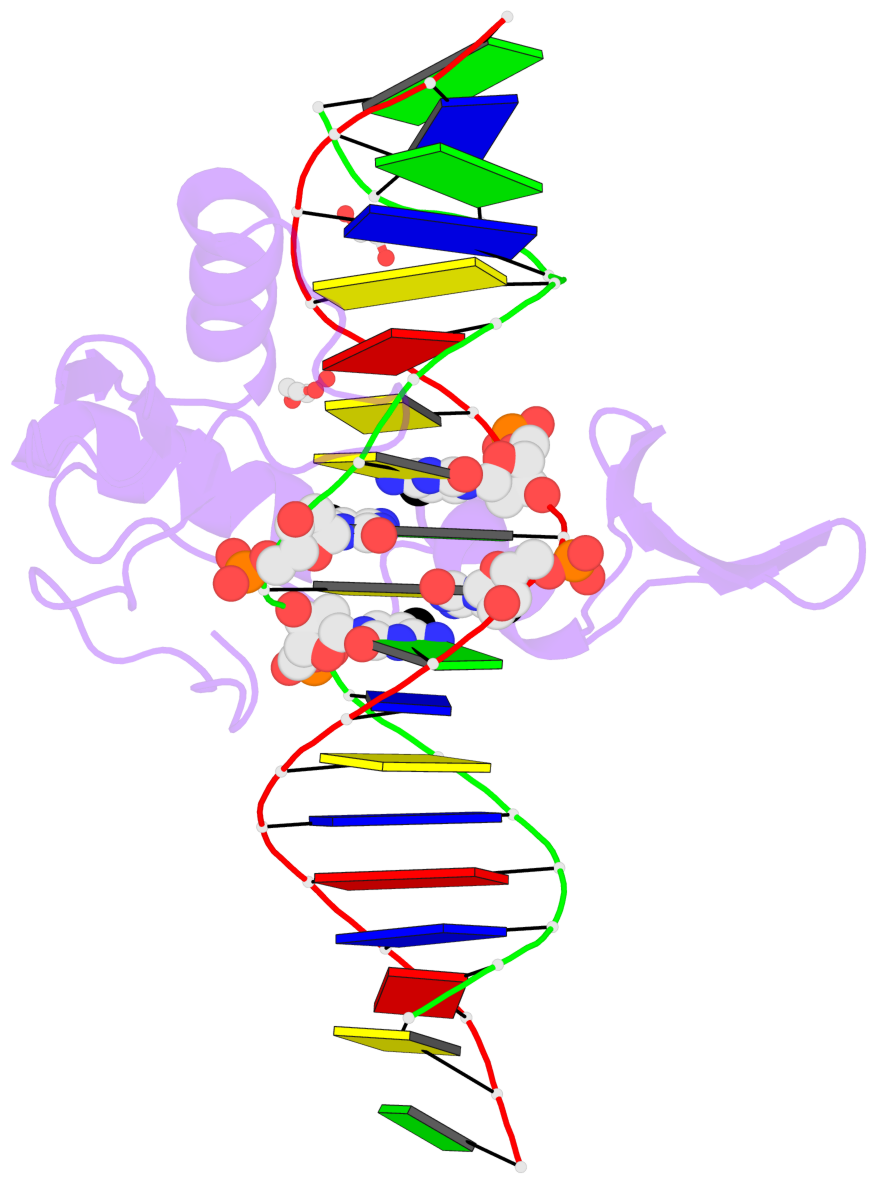
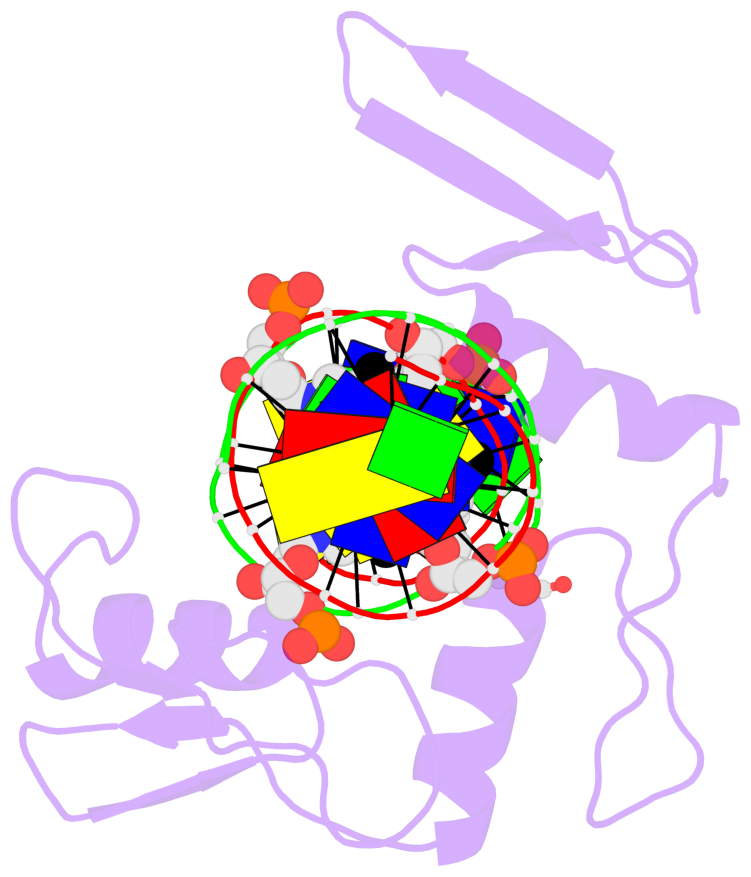
Summary information and primary citation
- PDB-id
- 4f6n; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.8 Å)
- Summary
- Crystal structure of kaiso zinc finger DNA binding protein in complex with methylated cpg site DNA
- Reference
- Buck-Koehntop BA, Stanfield RL, Ekiert DC, Martinez-Yamout MA, Dyson HJ, Wilson IA, Wright PE (2012): "Molecular basis for recognition of methylated and specific DNA sequences by the zinc finger protein Kaiso." Proc.Natl.Acad.Sci.USA, 109, 15229-15234. doi: 10.1073/pnas.1213726109.
- Abstract
- Methylation of CpG dinucleotides in DNA is a common epigenetic modification in eukaryotes that plays a central role in maintenance of genome stability, gene silencing, genomic imprinting, development, and disease. Kaiso, a bifunctional Cys(2)His(2) zinc finger protein implicated in tumor-cell proliferation, binds to both methylated CpG (mCpG) sites and a specific nonmethylated DNA motif (TCCTGCNA) and represses transcription by recruiting chromatin remodeling corepression machinery to target genes. Here we report structures of the Kaiso zinc finger DNA-binding domain in complex with its nonmethylated, sequence-specific DNA target (KBS) and with a symmetrically methylated DNA sequence derived from the promoter region of E-cadherin. Recognition of specific bases in the major groove of the core KBS and mCpG sites is accomplished through both classical and methyl CH···O hydrogen-bonding interactions with residues in the first two zinc fingers, whereas residues in the C-terminal extension following the third zinc finger bind in the opposing minor groove and are required for high-affinity binding. The C-terminal region is disordered in the free protein and adopts an ordered structure upon binding to DNA. The structures of these Kaiso complexes provide insights into the mechanism by which a zinc finger protein can recognize mCpG sites as well as a specific, nonmethylated regulatory DNA sequence.
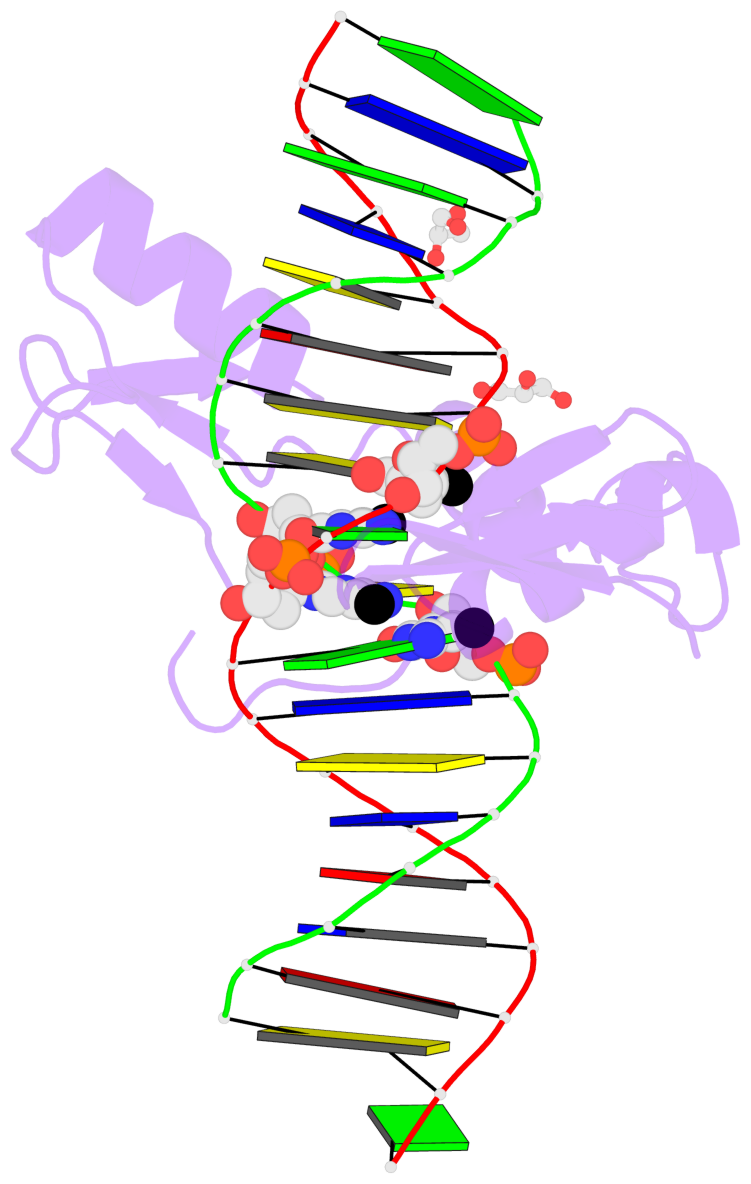
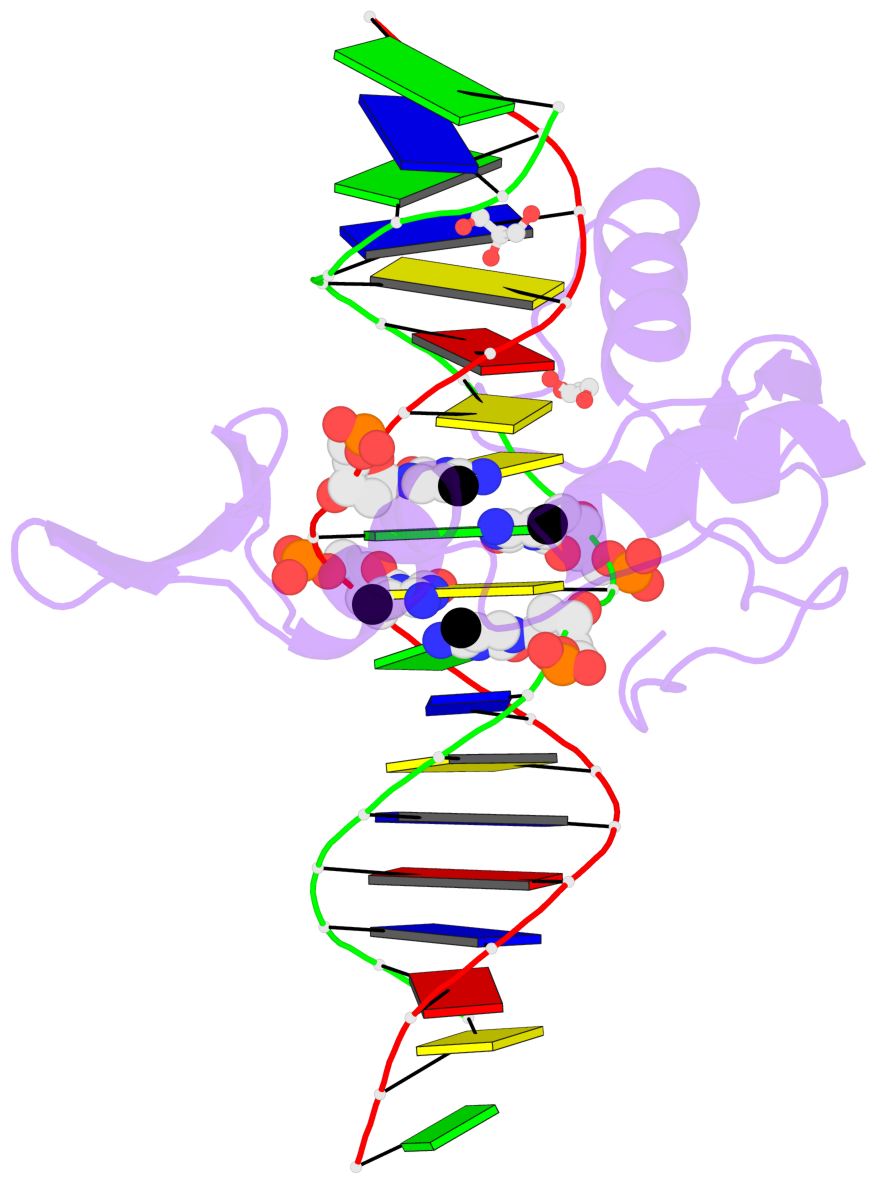
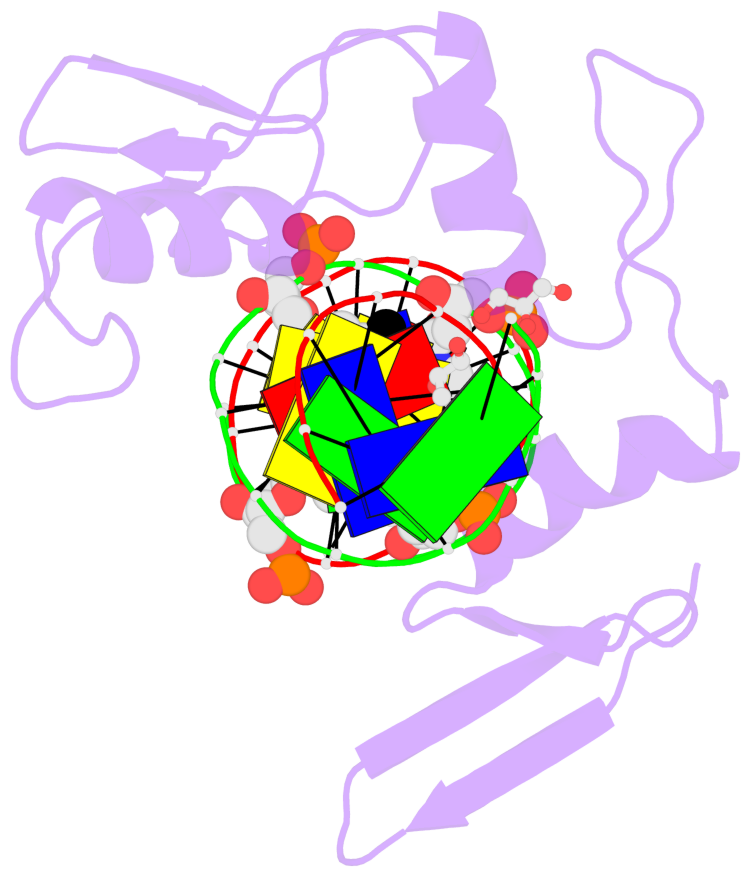
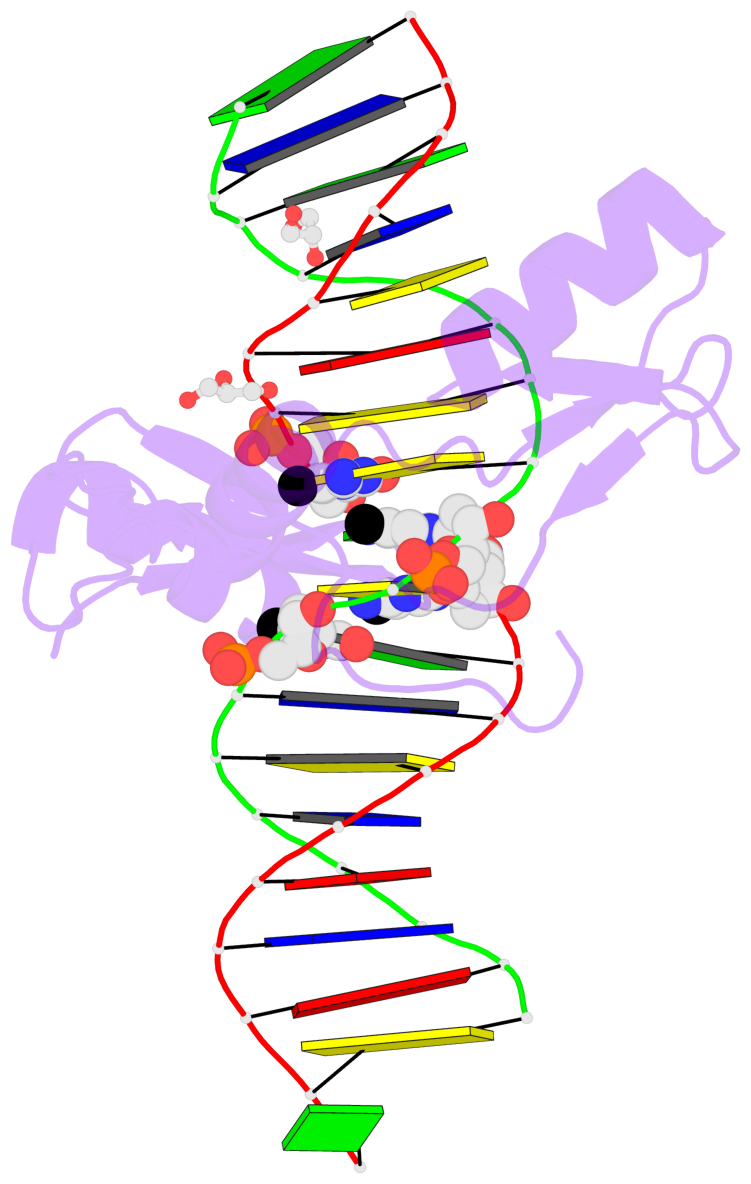
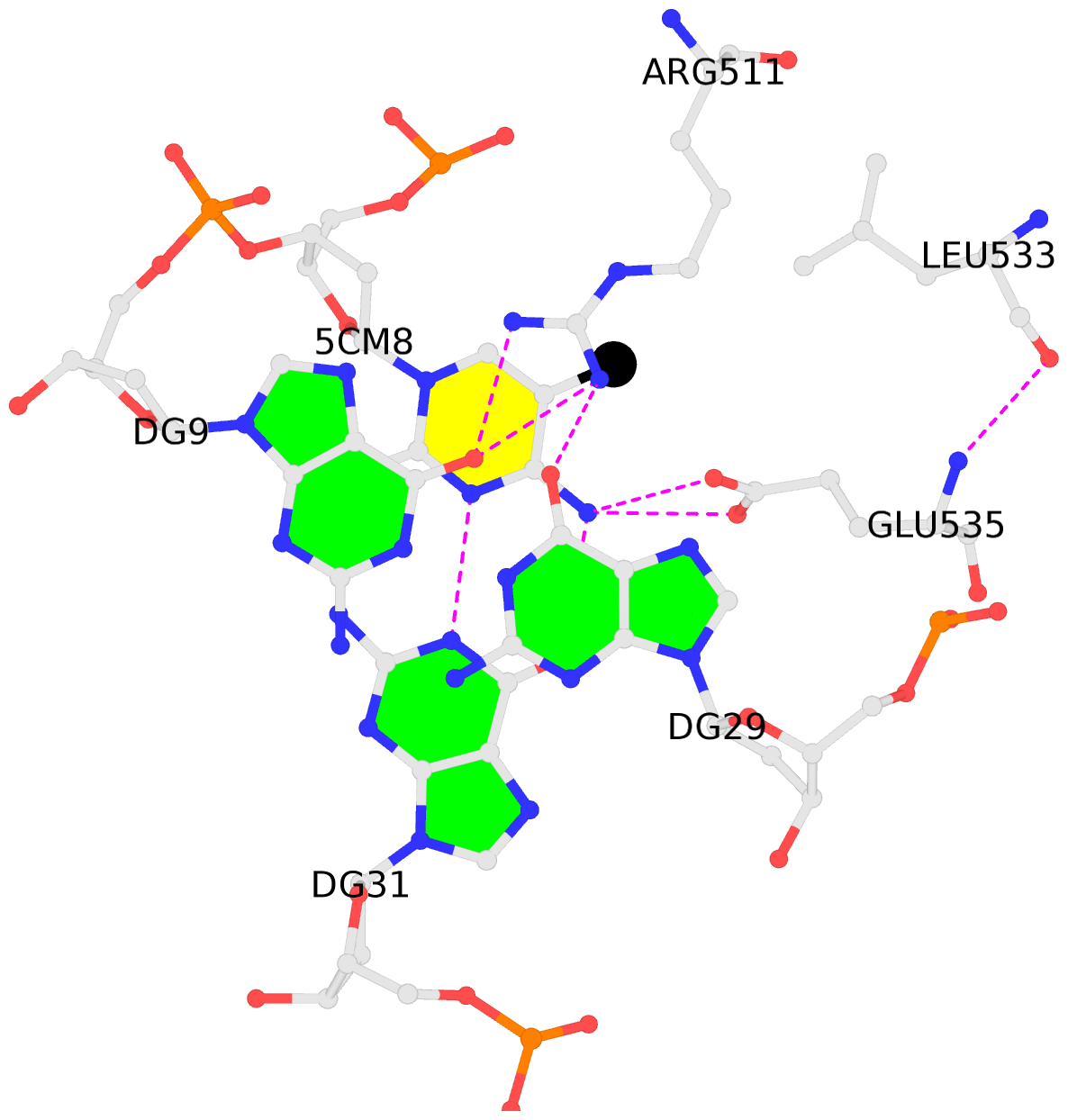
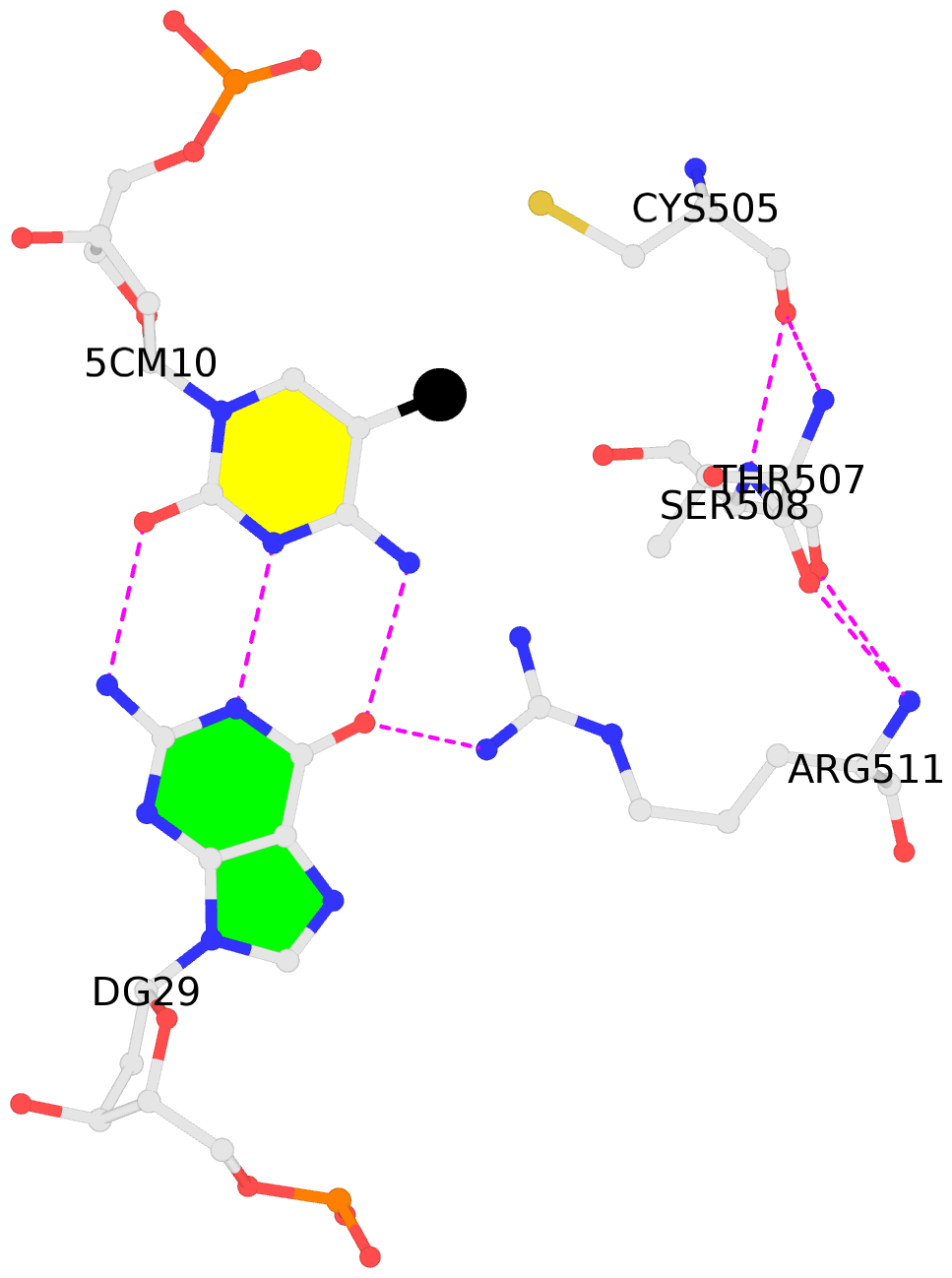
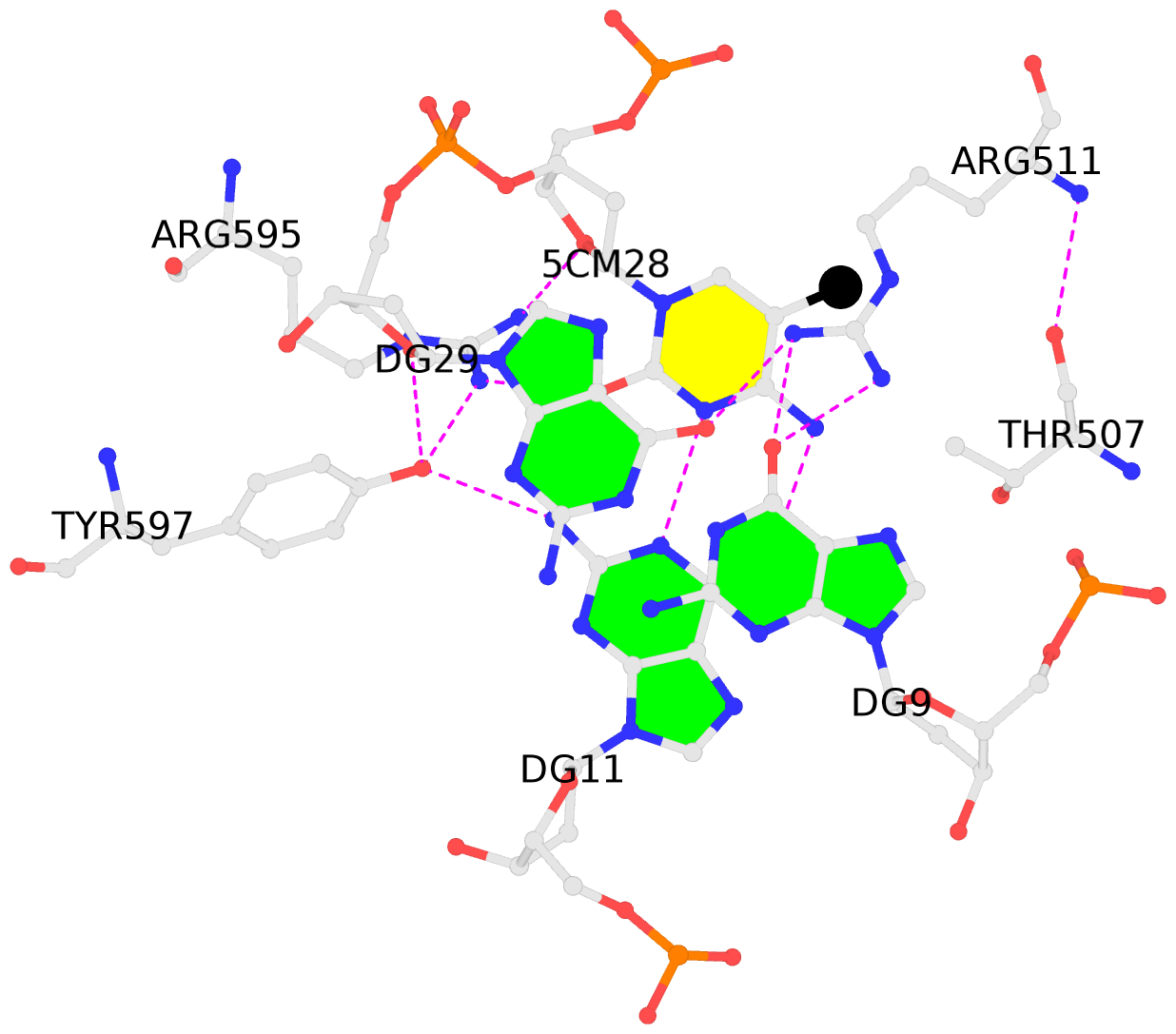
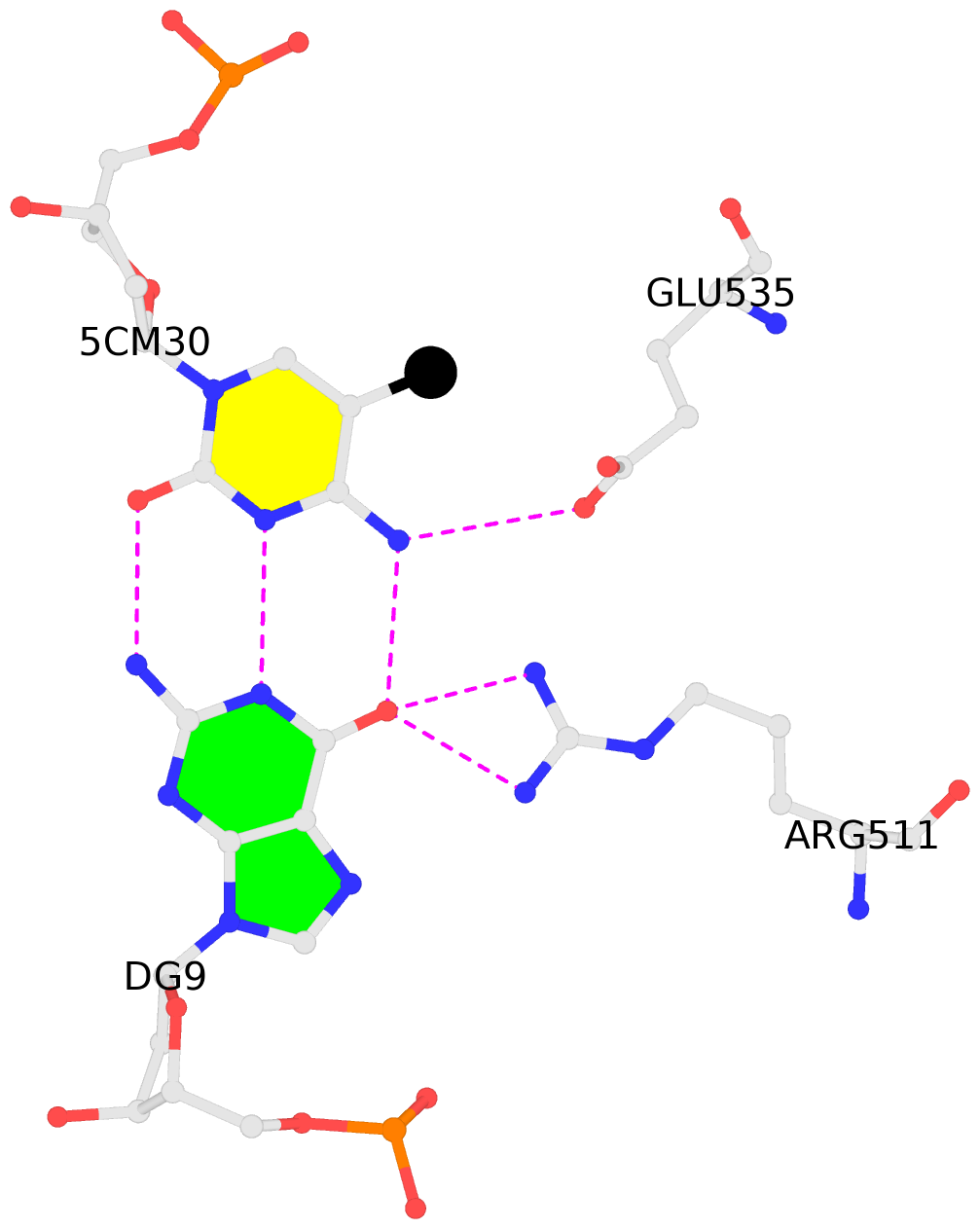
- The contacts include paired nucleotides (mostly a G in Watson-Crick G-C pairing), and
amino-acids within a 4.5-A distance cutoff to base atoms of 5mC.
- The structure is oriented in the base reference frame of 5mC, allowing for easy comparison
and direct superimposition between entries.
- The black sphere (•) denotes the 5-methyl carbon atom in 5mC.
No. 1 D.5CM8: stacking-with-A.ARG511 is-WC-paired is-in-duplex [+]:CcG/cGG |
 |
|
No. 2 D.5CM10: other-contacts is-WC-paired is-in-duplex [+]:GcG/cGc |
 |
|
No. 3 E.5CM28: stacking-with-A.ARG511 is-WC-paired is-in-duplex [-]:cGT/AcG |
 |
|
No. 4 E.5CM30: other-contacts is-WC-paired is-in-duplex [-]:cGc/GcG |
 |
|