Summary information and primary citation
- PDB-id
- 6mg2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.928 Å)
- Summary
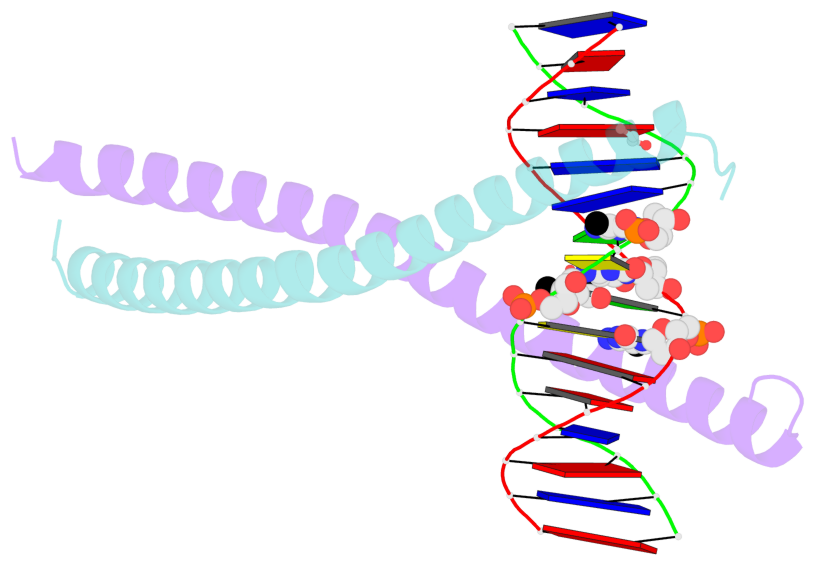
- C-terminal bzip domain of human c-ebpbeta with 16bp methylated oligonucleotide containing consensus recognition sequence-c2221 crystal form
- Reference
- Yang J, Horton JR, Wang D, Ren R, Li J, Sun D, Huang Y, Zhang X, Blumenthal RM, Cheng X (2019): "Structural basis for effects of CpA modifications on C/EBP beta binding of DNA." Nucleic Acids Res., 47, 1774-1785. doi: 10.1093/nar/gky1264.
- Abstract
- CCAAT/enhancer binding proteins (C/EBPs) regulate gene expression in a variety of cells/tissues/organs, during a range of developmental stages, under both physiological and pathological conditions. C/EBP-related transcription factors have a consensus binding specificity of 5'-TTG-CG-CAA-3', with a central CpG/CpG and two outer CpA/TpG dinucleotides. Methylation of the CpG and CpA sites generates a DNA element with every pyrimidine having a methyl group in the 5-carbon position (thymine or 5-methylcytosine (5mC)). To understand the effects of both CpG and CpA modification on a centrally-important transcription factor, we show that C/EBPβ binds the methylated 8-bp element with modestly-increased (2.4-fold) binding affinity relative to the unmodified cognate sequence, while cytosine hydroxymethylation (particularly at the CpA sites) substantially decreased binding affinity (36-fold). The structure of C/EBPβ DNA binding domain in complex with methylated DNA revealed that the methyl groups of the 5mCpA/TpG make van der Waals contacts with Val285 in C/EBPβ. Arg289 recognizes the central 5mCpG by forming a methyl-Arg-G triad, and its conformation is constrained by Val285 and the 5mCpG methyl group. We substituted Val285 with Ala (V285A) in an Ala-Val dipeptide, to mimic the conserved Ala-Ala in many members of the basic leucine-zipper family of transcription factors, important in gene regulation, cell proliferation and oncogenesis. The V285A variant demonstrated a 90-fold binding preference for methylated DNA (particularly 5mCpA methylation) over the unmodified sequence. The smaller side chain of Ala285 permits Arg289 to adopt two alternative conformations, to interact in a similar fashion with either the central 5mCpG or the TpG of the opposite strand. Significantly, the best-studied cis-regulatory elements in RNA polymerase II promoters and enhancers have variable sequences corresponding to the central CpG or reduced to a single G:C base pair, but retain a conserved outer CpA sequence. Our analyses suggest an important modification-dependent CpA recognition by basic leucine-zipper transcription factors.
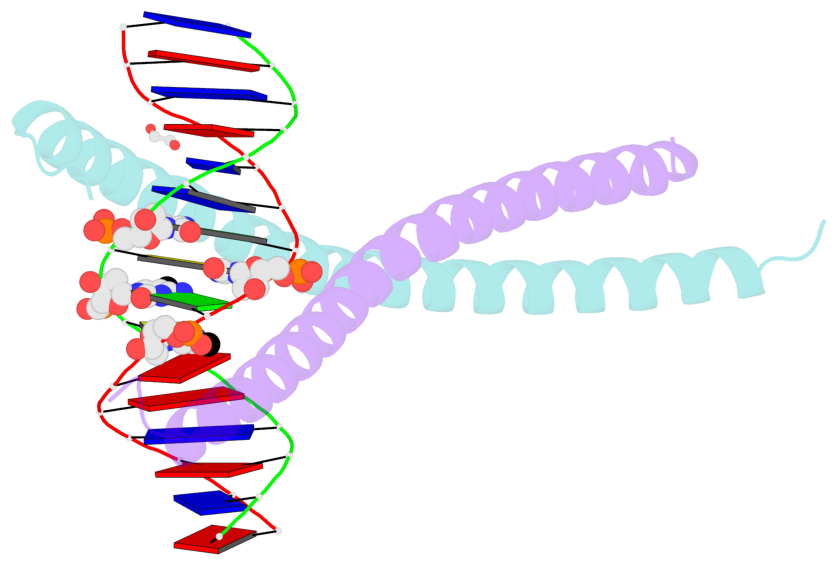
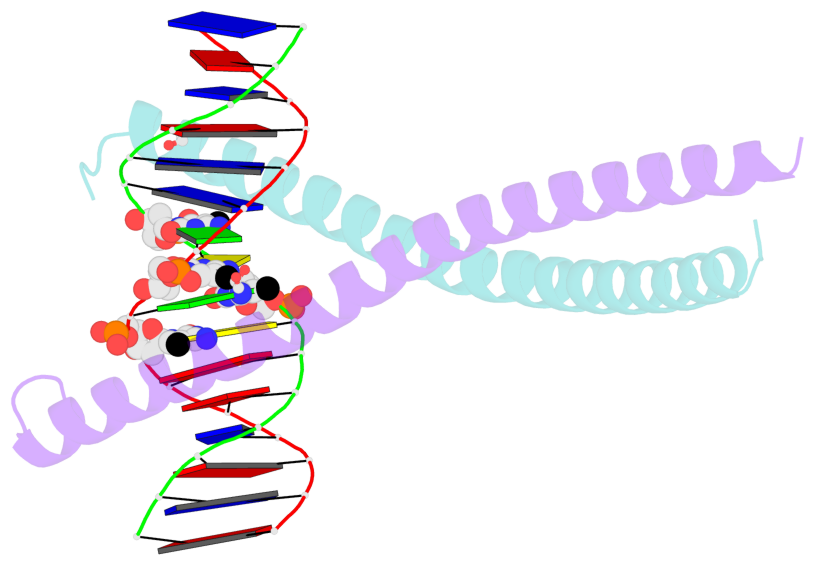
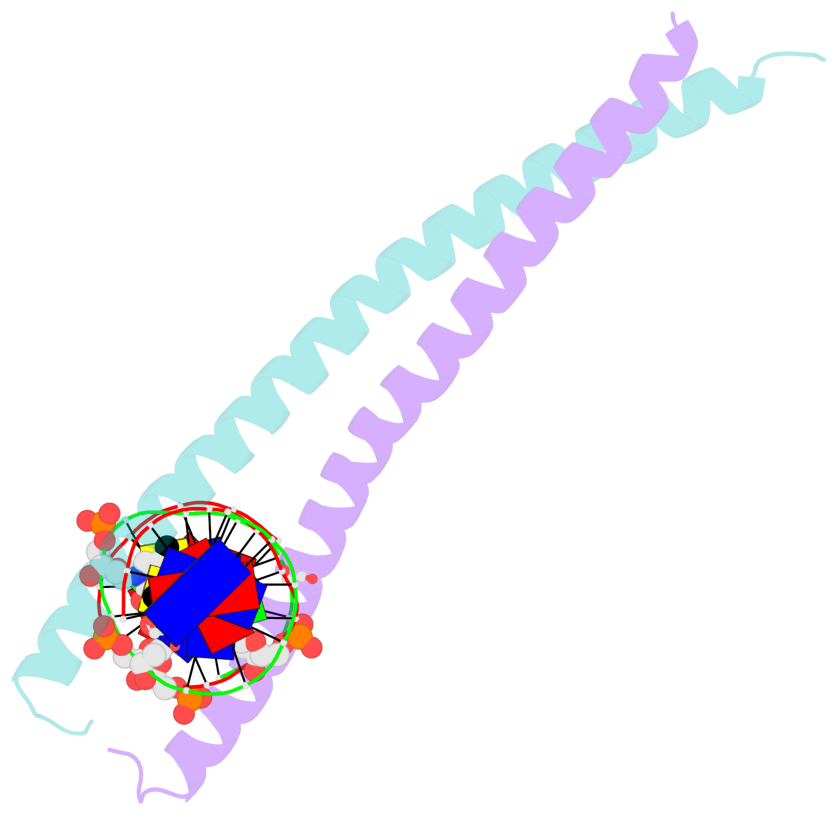
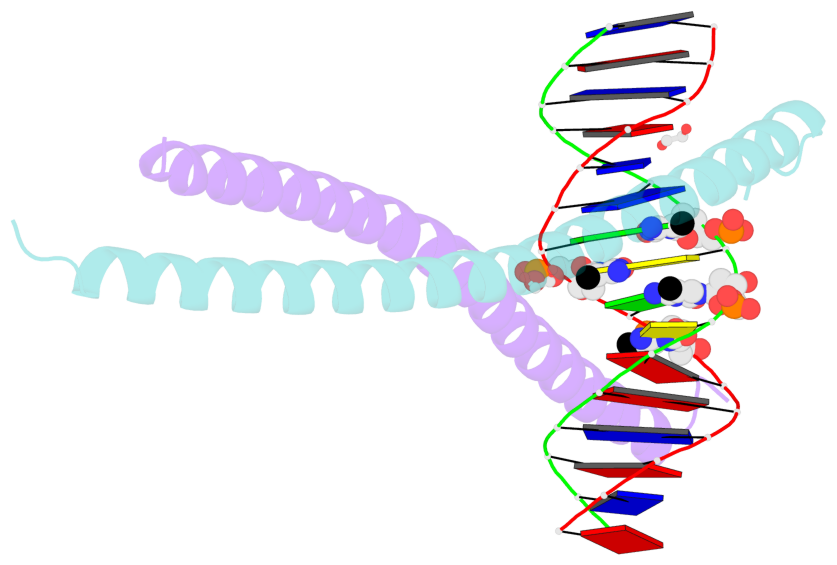
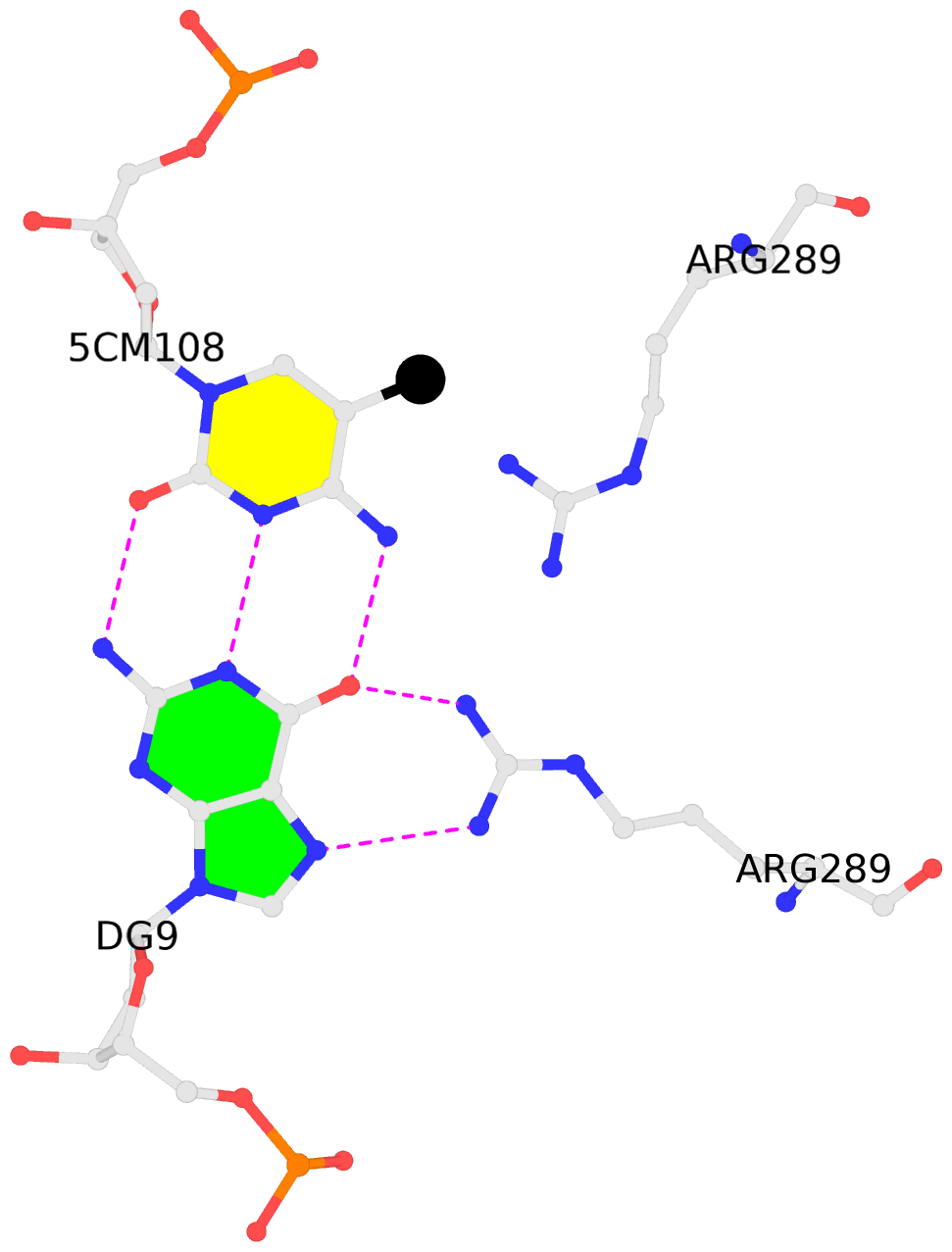
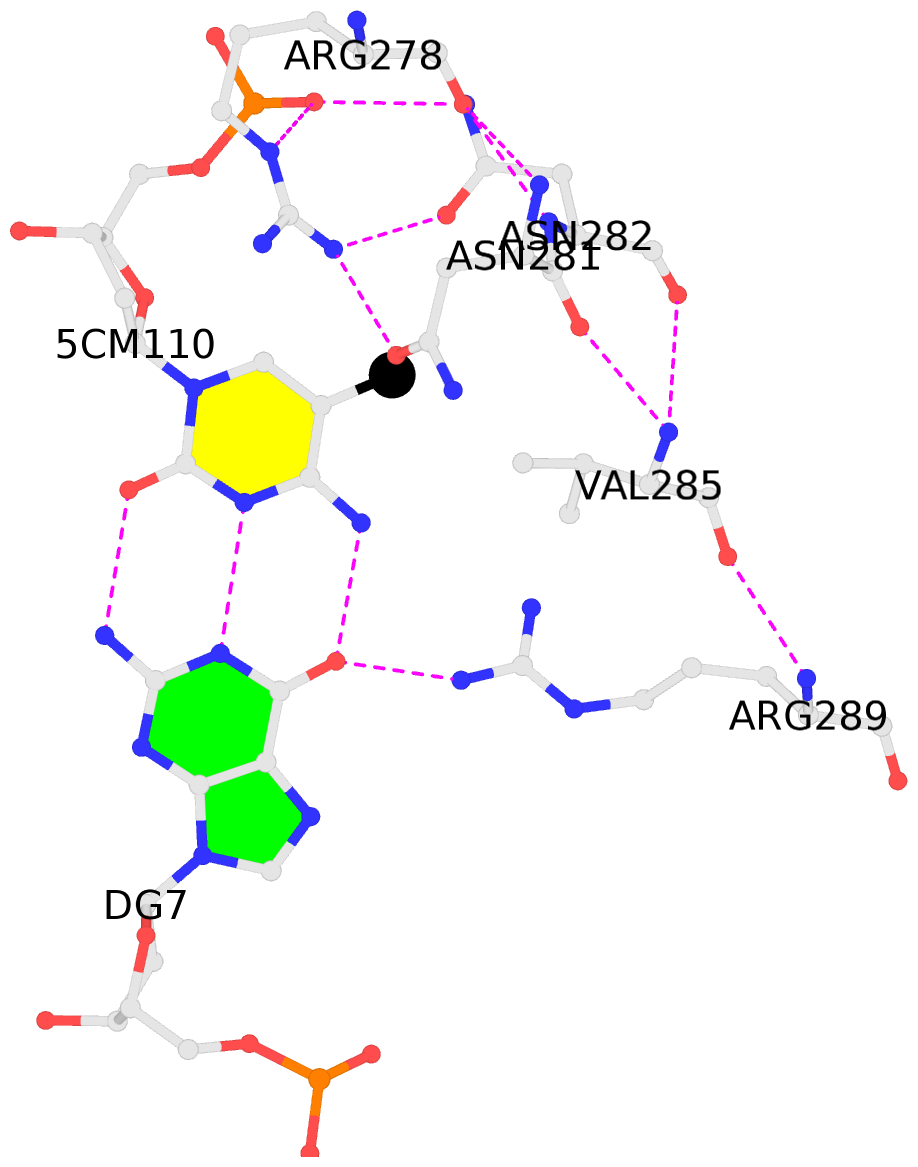
- The contacts include paired nucleotides (mostly a G in Watson-Crick G-C pairing), and
amino-acids within a 4.5-A distance cutoff to base atoms of 5mC.
- The structure is oriented in the base reference frame of 5mC, allowing for easy comparison
and direct superimposition between entries.
- The black sphere (•) denotes the 5-methyl carbon atom in 5mC.
No. 1 C.5CM8: stacking-with-A.ARG289 is-WC-paired is-in-duplex [+]:GcG/cGc |
 |
|
No. 2 C.5CM10: hydrophobic-with-A.VAL285 is-WC-paired is-in-duplex [+]:GcA/TGc |
 |
|
No. 3 D.5CM108: other-contacts is-WC-paired is-in-duplex [-]:cGc/GcG |
 |
|
No. 4 D.5CM110: hydrophobic-with-B.VAL285 is-WC-paired is-in-duplex [-]:TGc/GcA |
 |
|