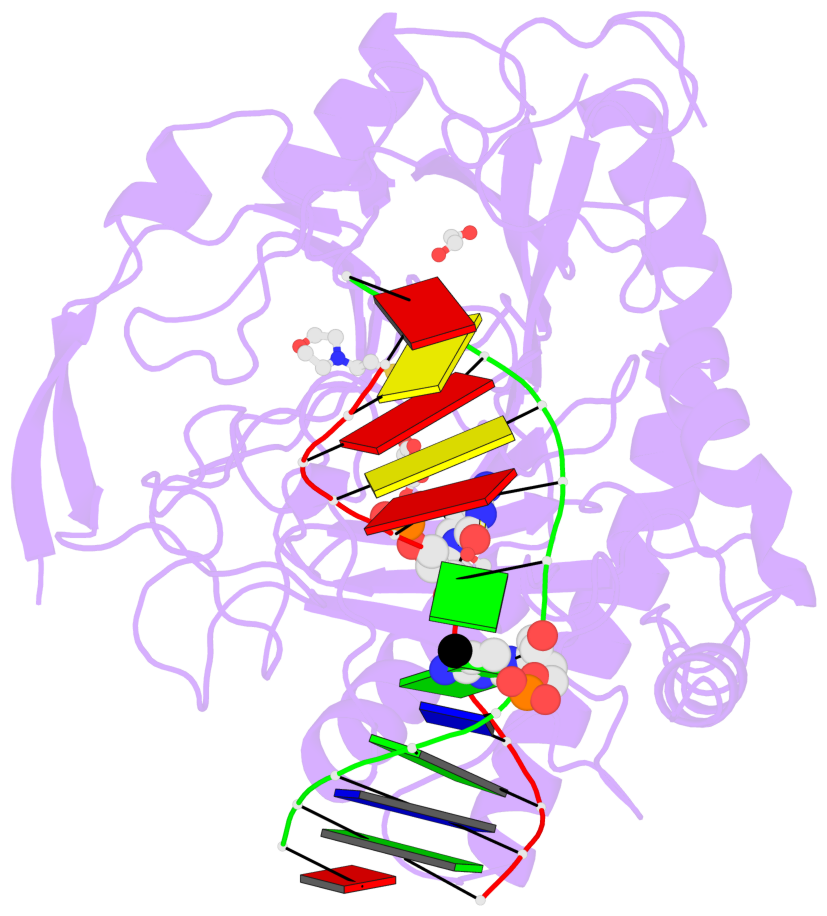
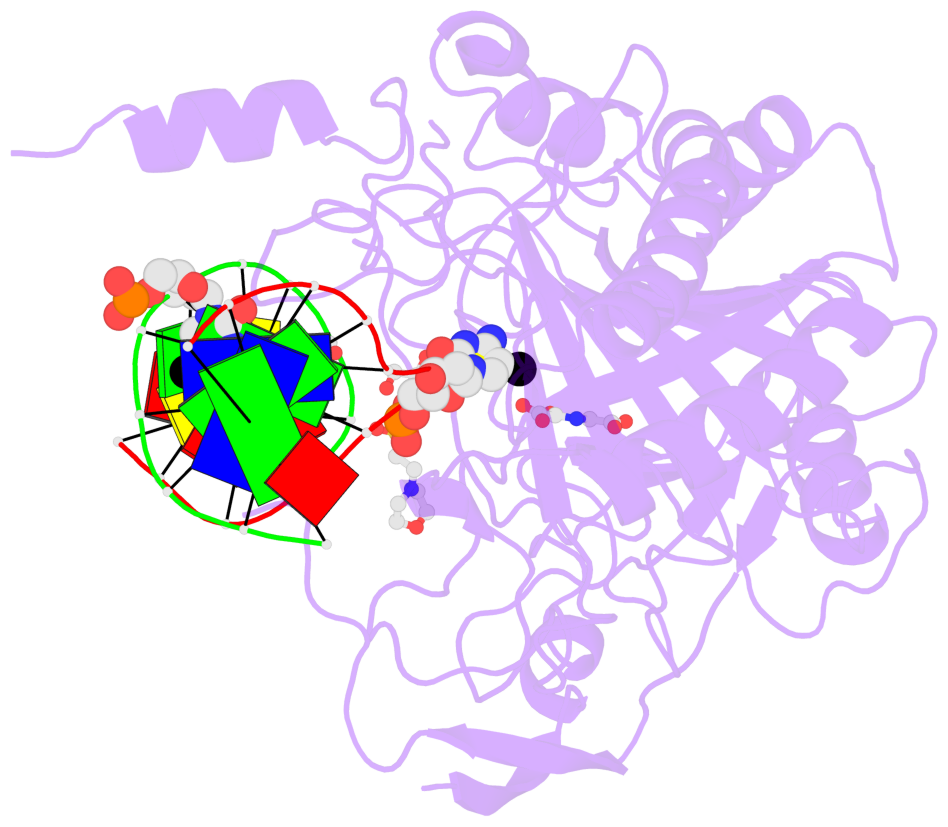
Summary information and primary citation
- PDB-id
- 7ne3; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- oxidoreductase
- Method
- X-ray (2.26 Å)
- Summary
- Human tet2 in complex with favourable DNA substrate.
- Reference
- Ravichandran M, Rafalski D, Davies CI, Ortega-Recalde O, Nan X, Glanfield CR, Kotter A, Misztal K, Wang AH, Wojciechowski M, Razew M, Mayyas IM, Kardailsky O, Schwartz U, Zembrzycki K, Morison IM, Helm M, Weichenhan D, Jurkowska RZ, Krueger F, Plass C, Zacharias M, Bochtler M, Hore TA, Jurkowski TP (2022): "Pronounced sequence specificity of the TET enzyme catalytic domain guides its cellular function." Sci Adv, 8, eabm2427. doi: 10.1126/sciadv.abm2427.
- Abstract
- TET (ten-eleven translocation) enzymes catalyze the oxidation of 5-methylcytosine bases in DNA, thus driving active and passive DNA demethylation. Here, we report that the catalytic domain of mammalian TET enzymes favor CGs embedded within basic helix-loop-helix and basic leucine zipper domain transcription factor-binding sites, with up to 250-fold preference in vitro. Crystal structures and molecular dynamics calculations show that sequence preference is caused by intrasubstrate interactions and CG flanking sequence indirectly affecting enzyme conformation. TET sequence preferences are physiologically relevant as they explain the rates of DNA demethylation in TET-rescue experiments in culture and in vivo within the zygote and germ line. Most and least favorable TET motifs represent DNA sites that are bound by methylation-sensitive immediate-early transcription factors and octamer-binding transcription factor 4 (OCT4), respectively, illuminating TET function in transcriptional responses and pluripotency support.
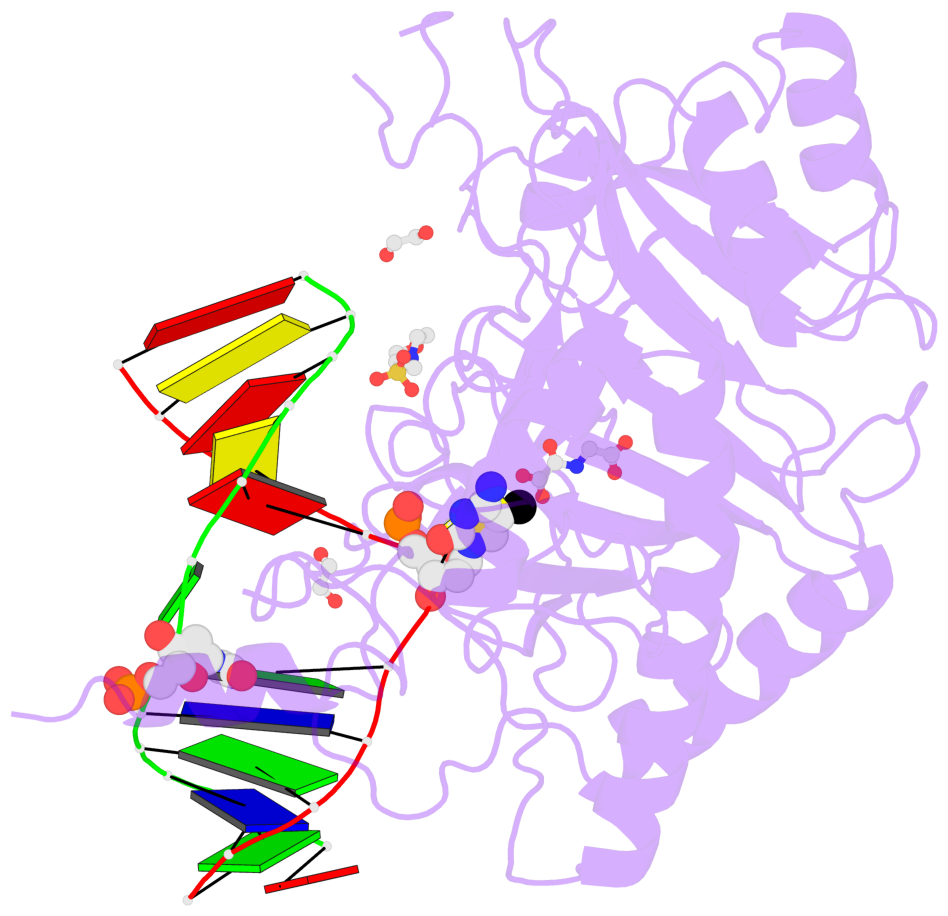
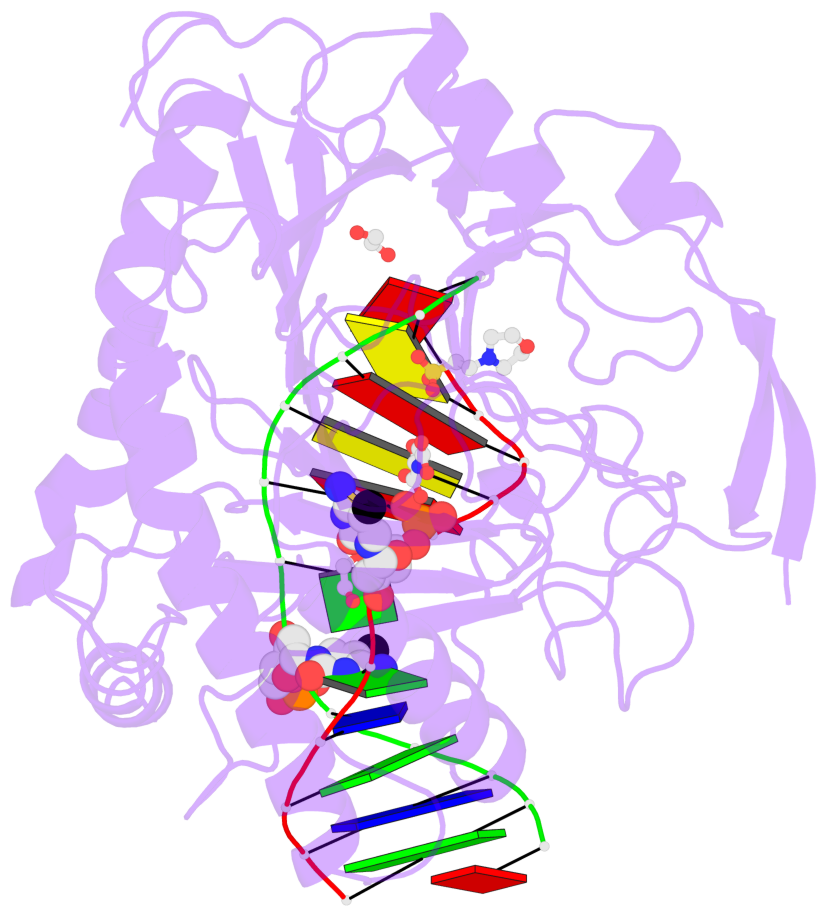
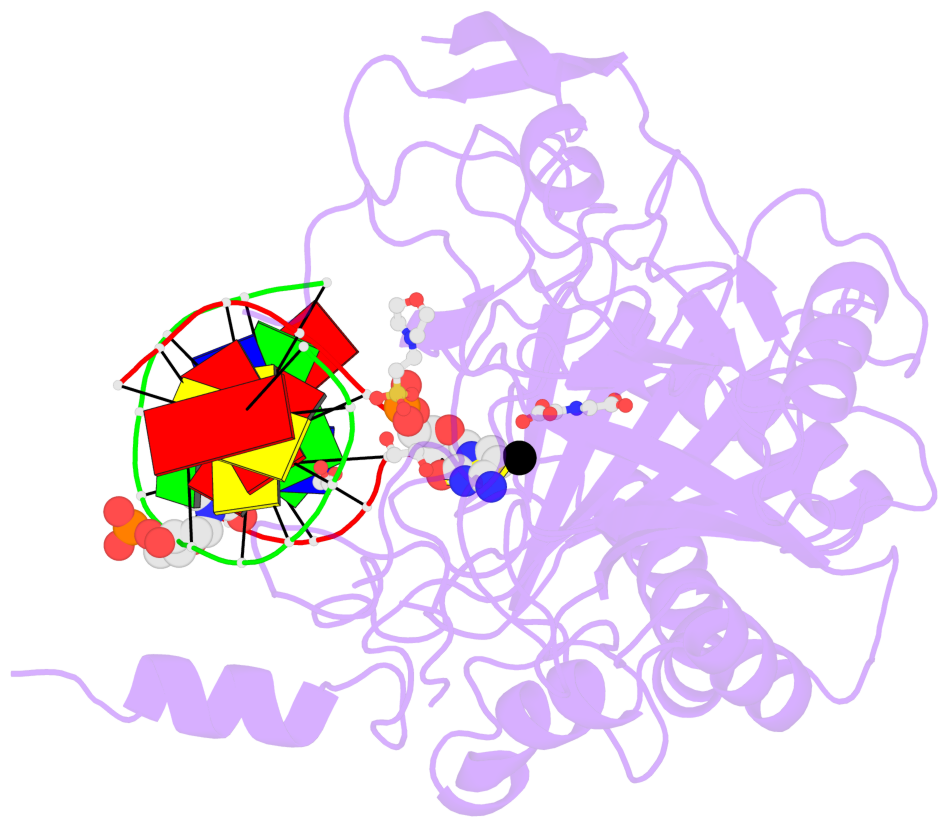
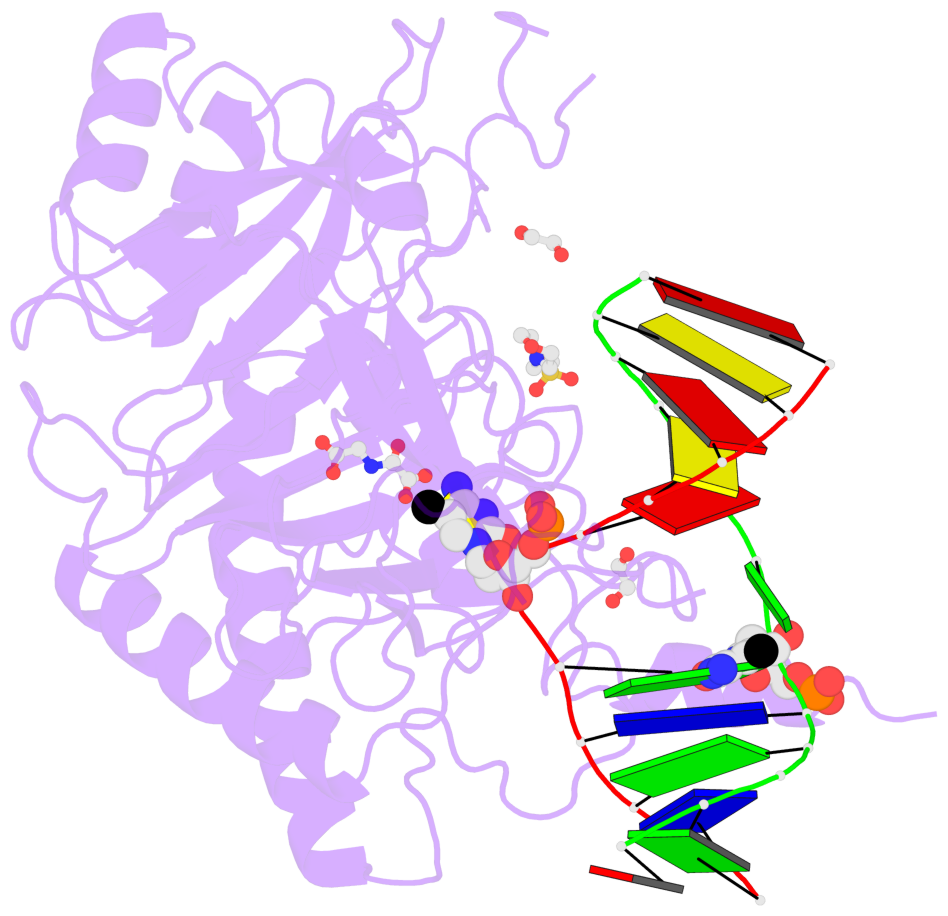
- The contacts include paired nucleotides (mostly a G in Watson-Crick G-C pairing), and
amino-acids within a 4.5-A distance cutoff to base atoms of 5mC.
- The structure is oriented in the base reference frame of 5mC, allowing for easy comparison
and direct superimposition between entries.
- The black sphere (•) denotes the 5-methyl carbon atom in 5mC.
No. 1 B.5CM6: stacking-with-A.ARG1261 stacking-with-A.ASP1384 stacking-with-A.TYR1902 not-WC-paired not-in-duplex |
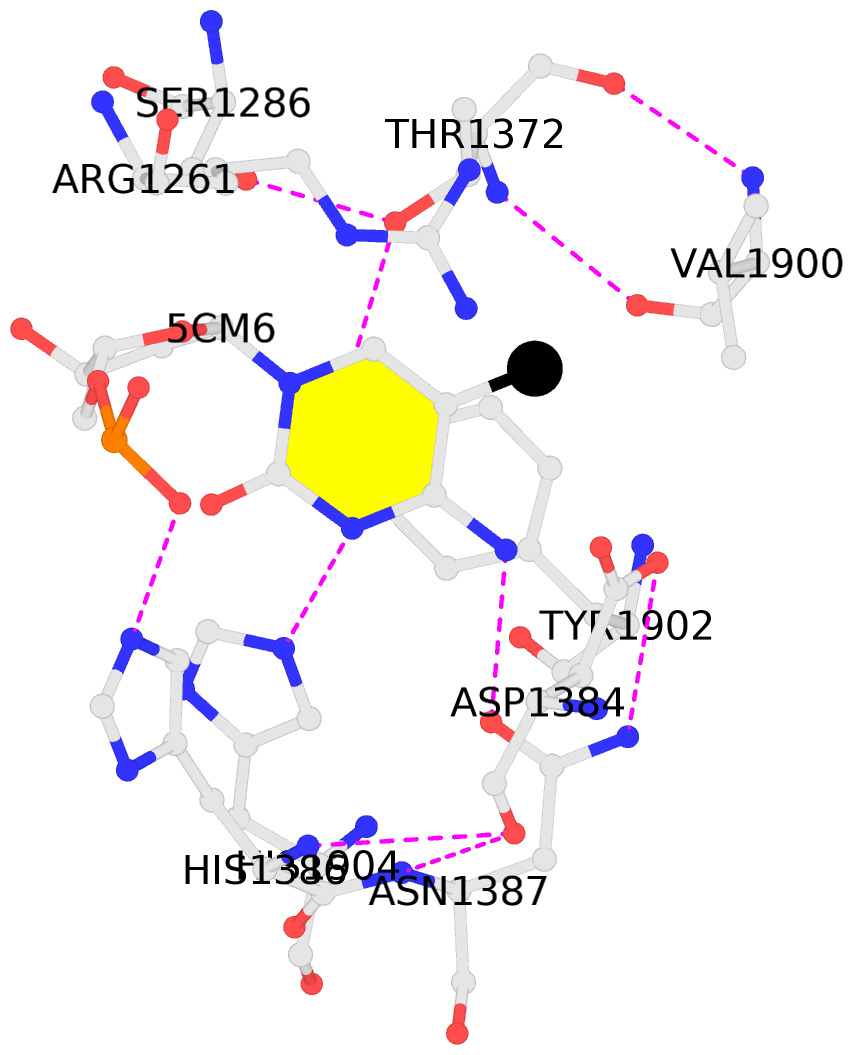 |
|
No. 2 C.5CM6: stacking-with-A.TYR1294 is-WC-paired is-in-duplex [-]:.GT/Ac. |
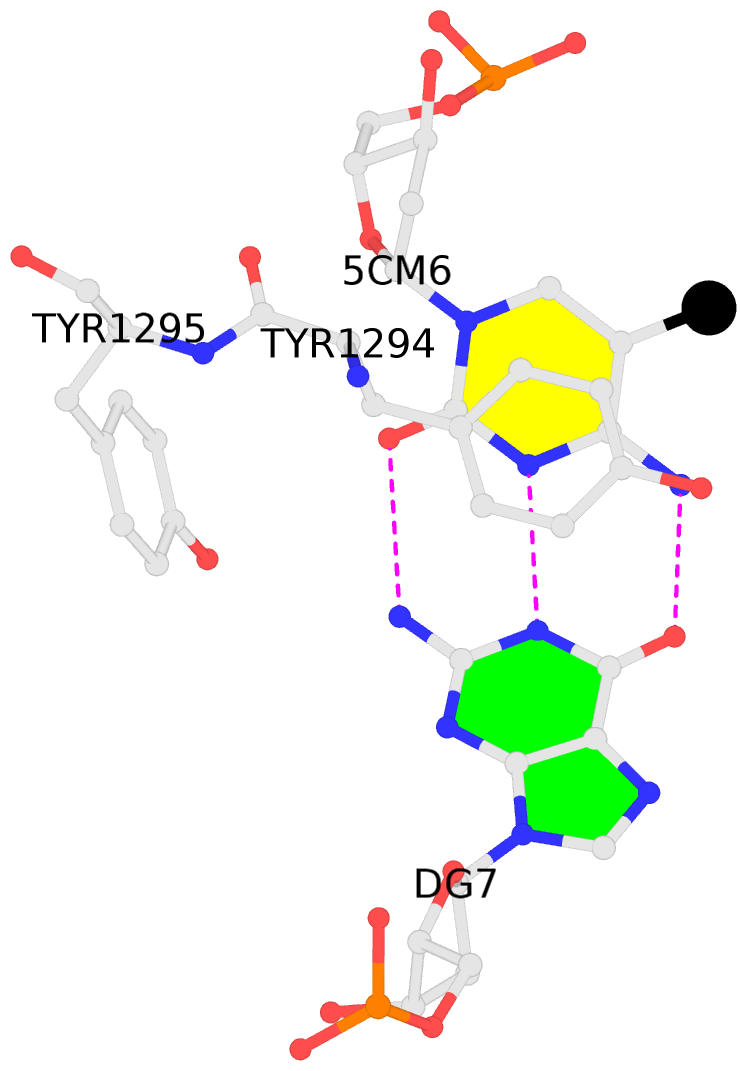 |
|