Summary information and primary citation
- PDB-id
- 7yhp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- cryo-EM (3.1 Å)
- Summary
- Cryoem structure of arabidopsis ros1 in complex with 5mc-dsDNA at 3.1 angstroms resolution
- Reference
- Du X, Yang Z, Xie G, Wang C, Zhang L, Yan K, Yang M, Li S, Zhu JK, Du J (2023): "Molecular basis of the plant ROS1-mediated active DNA demethylation." Nat.Plants, 9, 271-279. doi: 10.1038/s41477-022-01322-8.
- Abstract
- Active DNA demethylation plays a crucial role in eukaryotic gene imprinting and antagonizing DNA methylation. The plant-specific REPRESSOR OF SILENCING 1/DEMETER (ROS1/DME) family of enzymes directly excise 5-methyl-cytosine (5mC), representing an efficient DNA demethylation pathway distinct from that of animals. Here, we report the cryo-electron microscopy structures of an Arabidopsis ROS1 catalytic fragment in complex with substrate DNA, mismatch DNA and reaction intermediate, respectively. The substrate 5mC is flipped-out from the DNA duplex and subsequently recognized by the ROS1 base-binding pocket through hydrophobic and hydrogen-bonding interactions towards the 5-methyl group and Watson-Crick edge respectively, while the different protonation states of the bases determine the substrate preference for 5mC over T:G mismatch. Together with the structure of the reaction intermediate complex, our structural and biochemical studies revealed the molecular basis for substrate specificity, as well as the reaction mechanism underlying 5mC demethylation by the ROS1/DME family of plant-specific DNA demethylases.
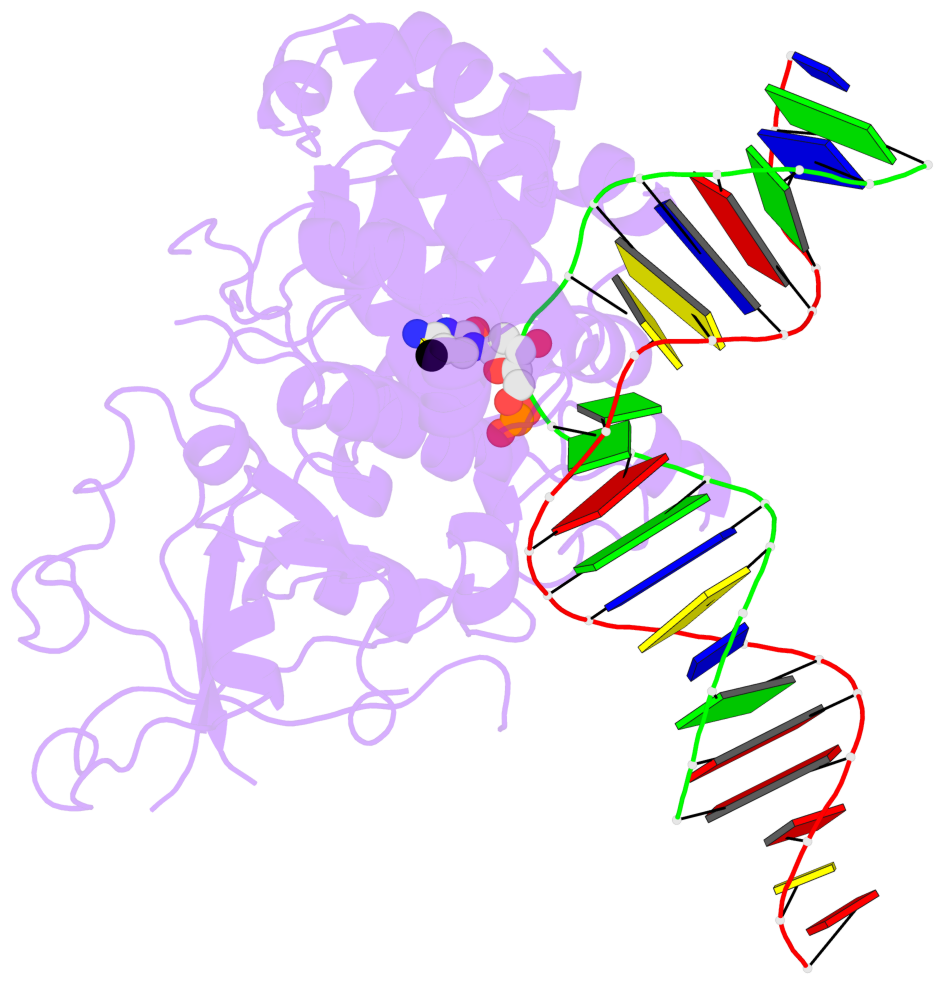
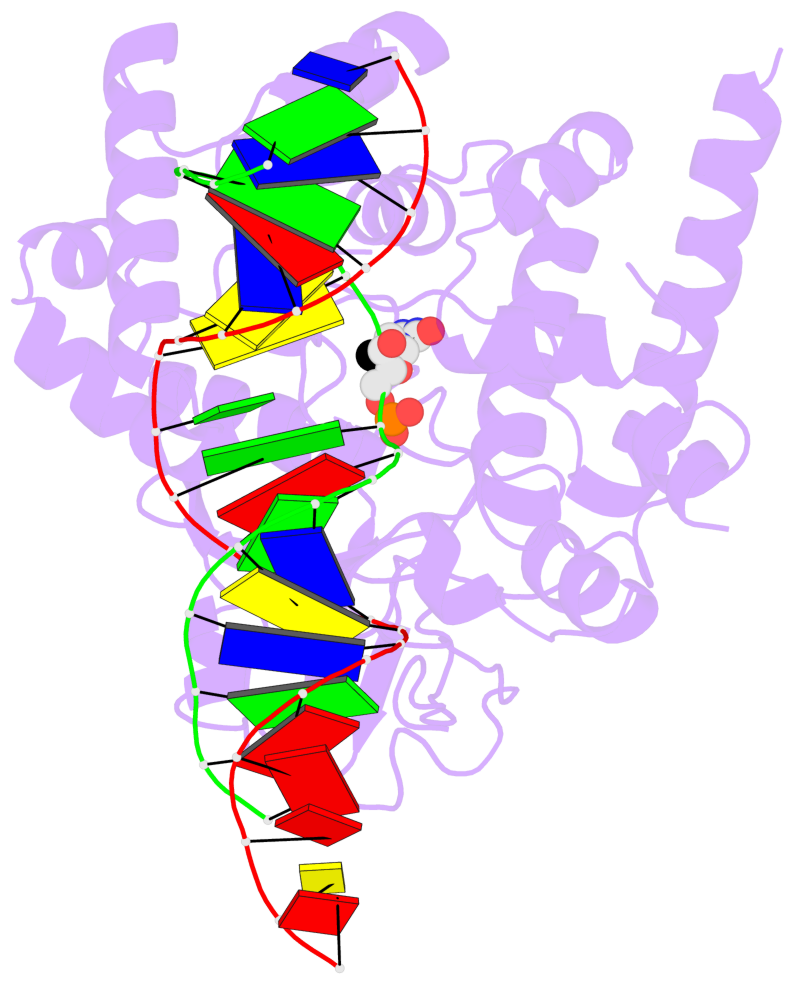
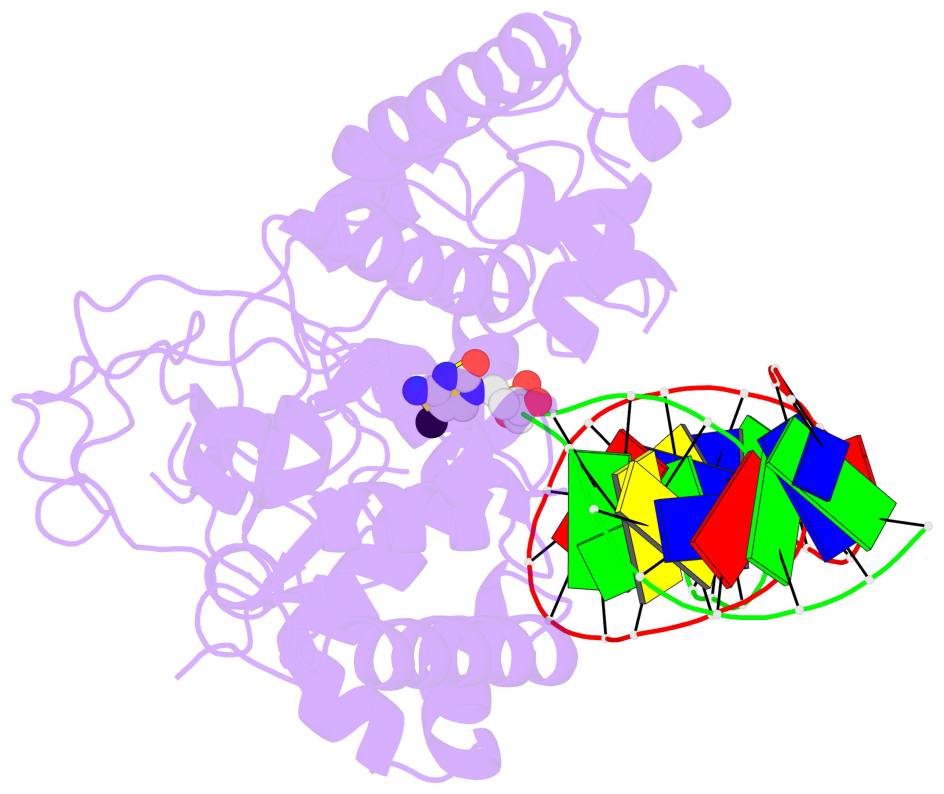
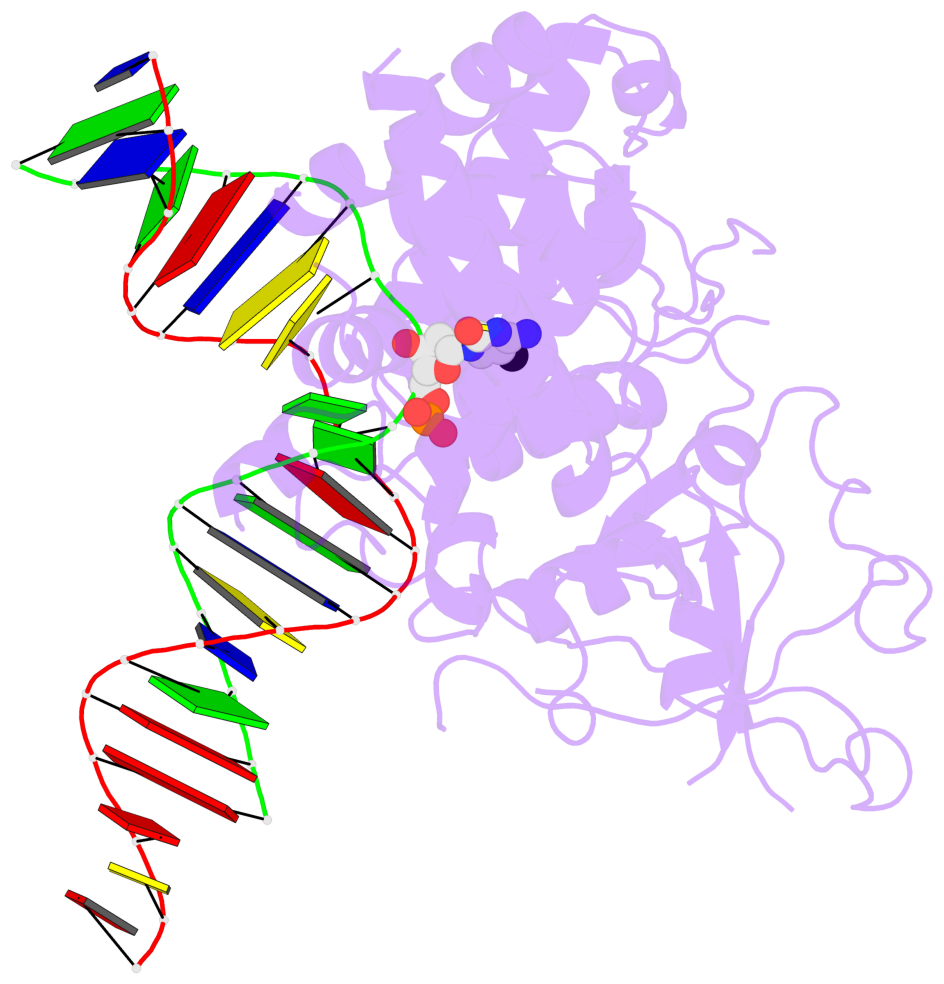
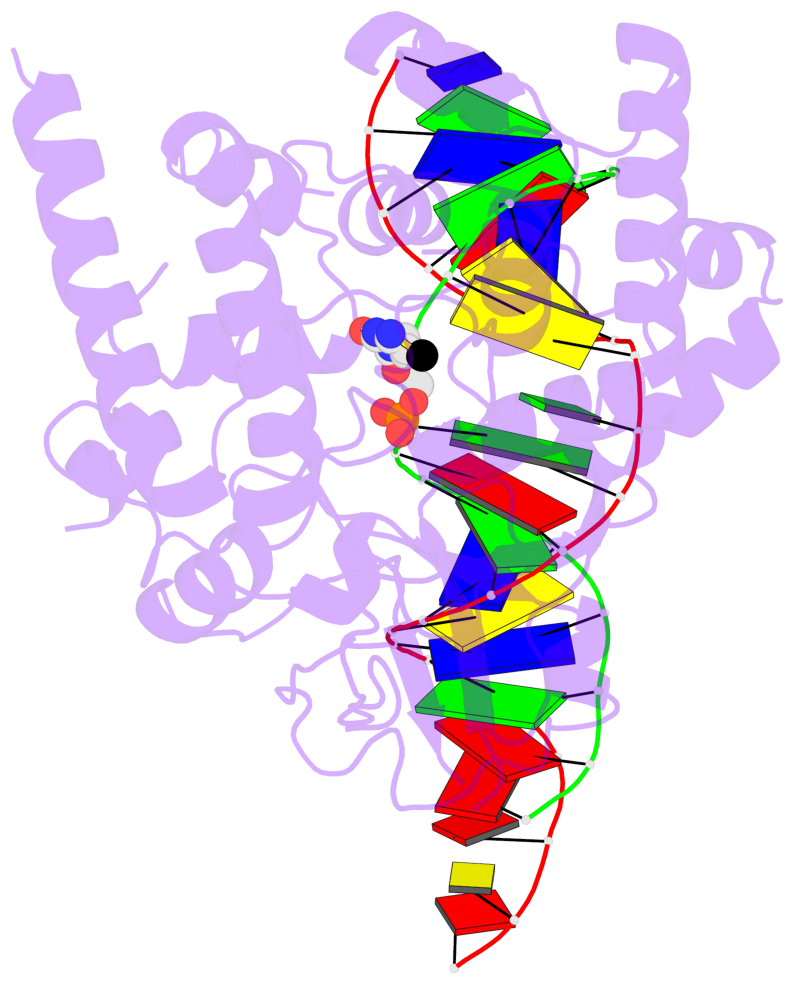
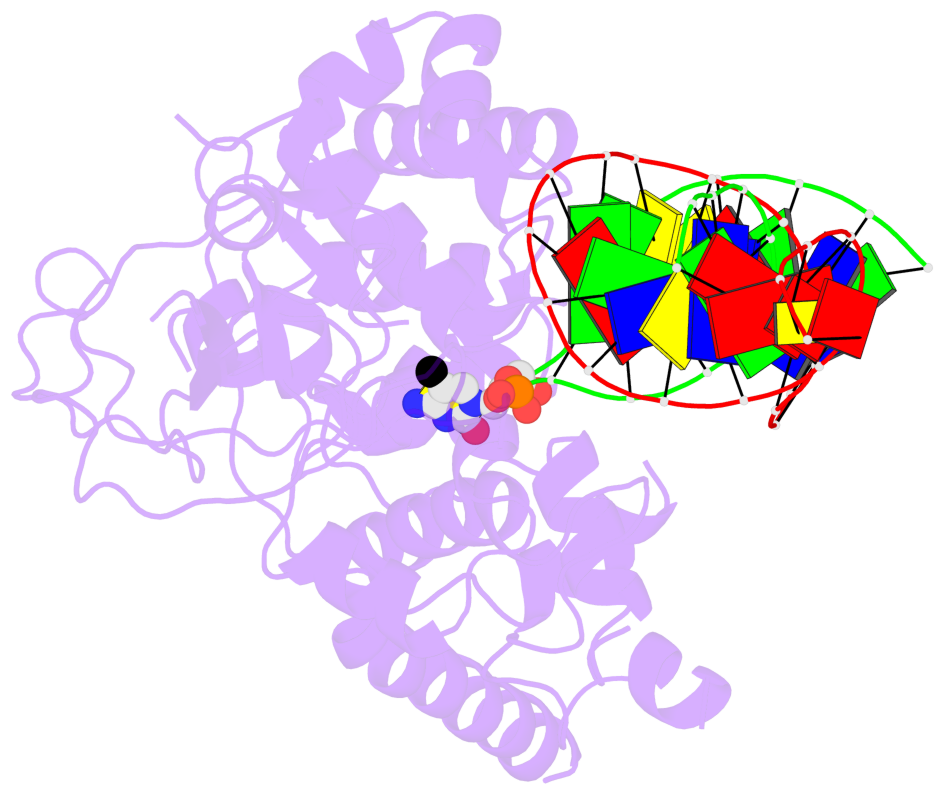
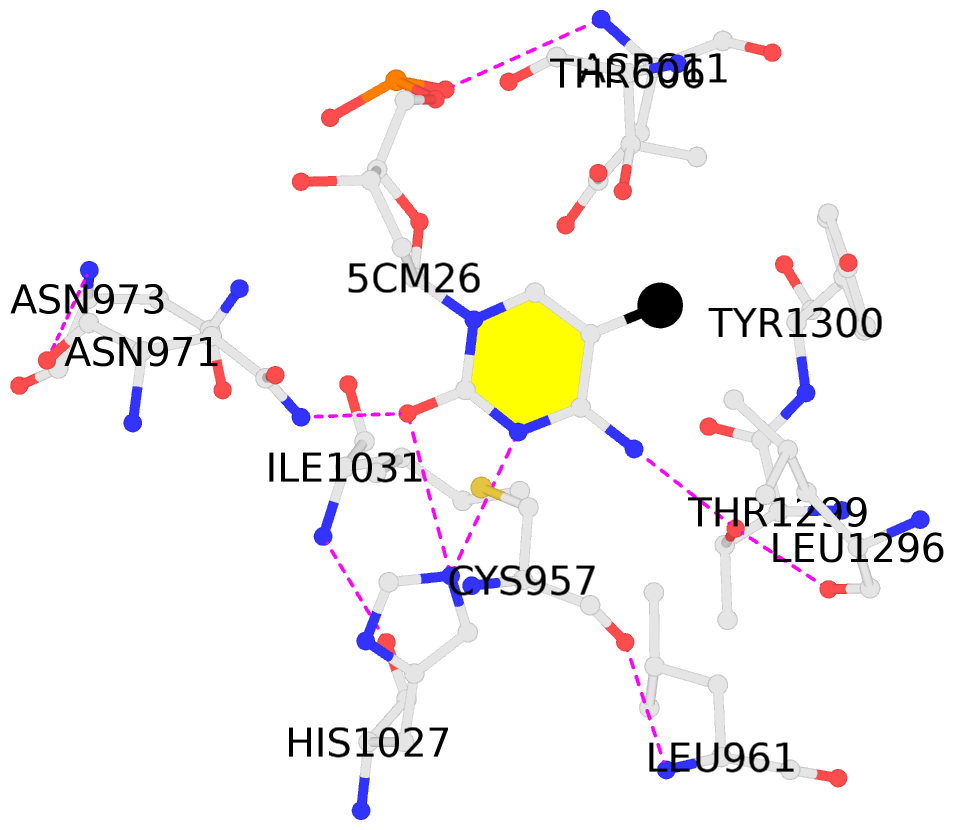
- The contacts include paired nucleotides (mostly a G in Watson-Crick G-C pairing), and
amino-acids within a 4.5-A distance cutoff to base atoms of 5mC.
- The structure is oriented in the base reference frame of 5mC, allowing for easy comparison
and direct superimposition between entries.
- The black sphere (•) denotes the 5-methyl carbon atom in 5mC.
No. 1 C.5CM26: hydrophobic-with-A.LEU961 hydrophobic-with-A.ILE1031 hydrophobic-with-A.LEU1296 not-WC-paired not-in-duplex |
 |
|