Summary information and primary citation
- PDB-id
- 1a31; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA
- Method
- X-ray (2.1 Å)
- Summary
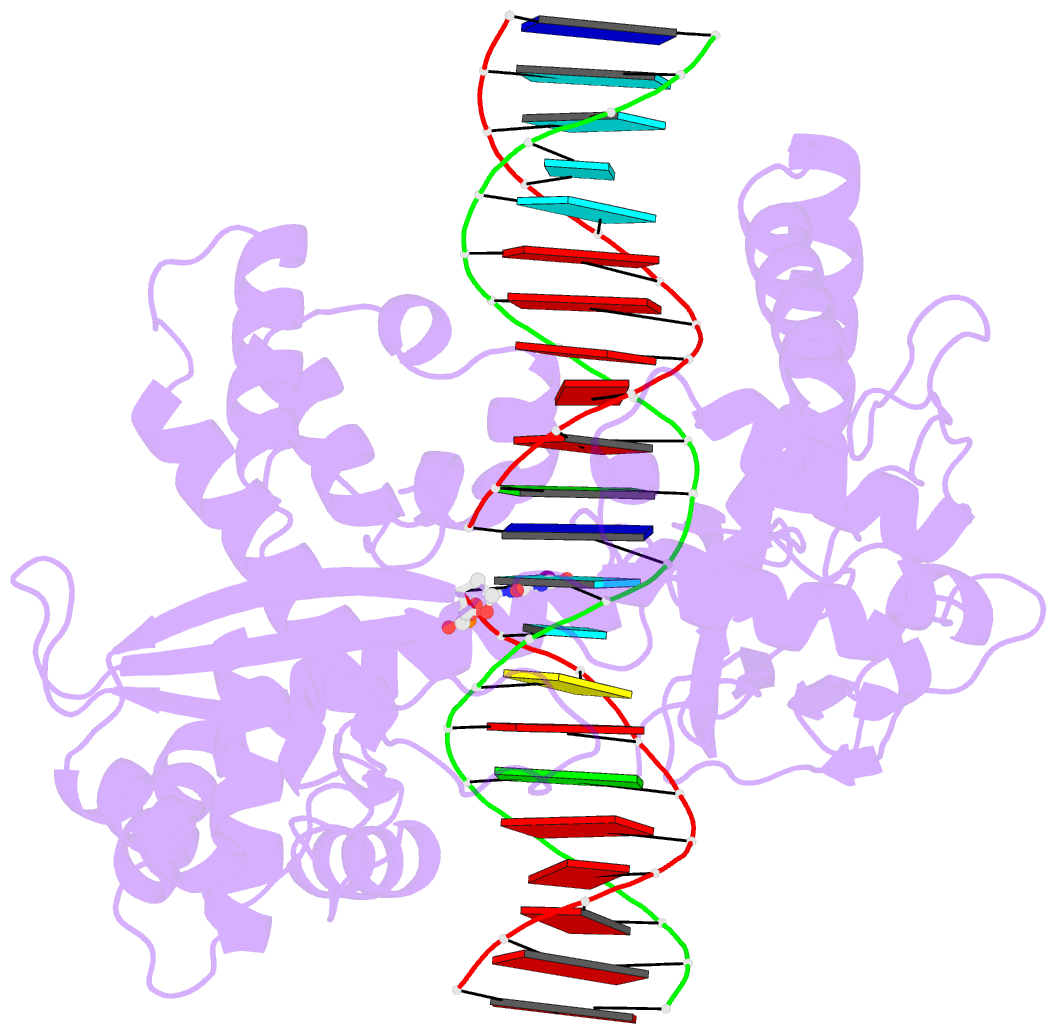
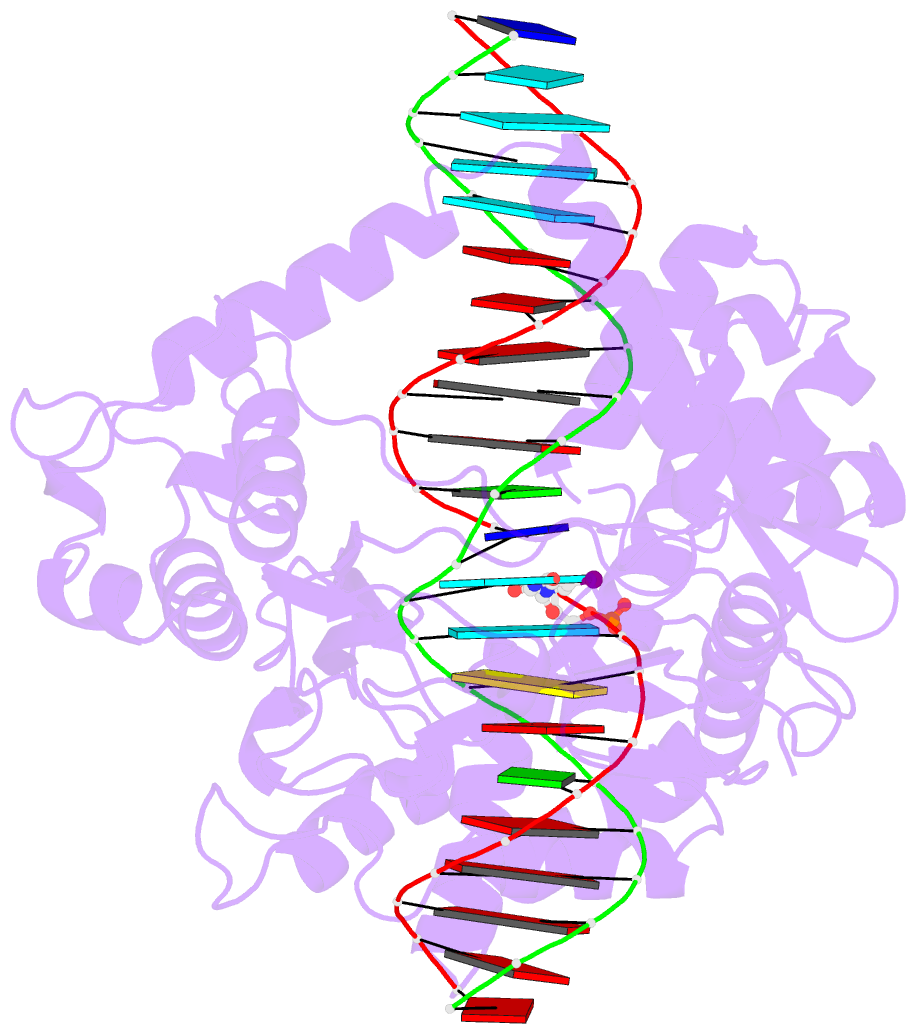
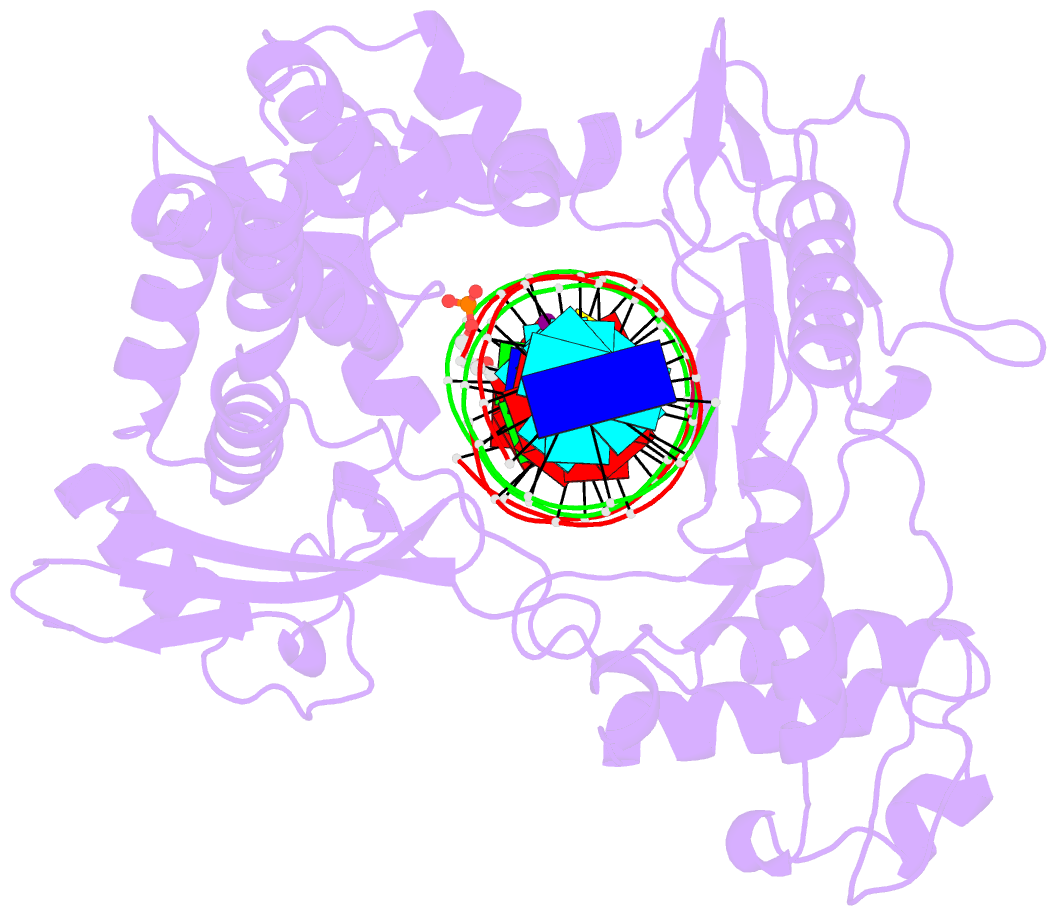
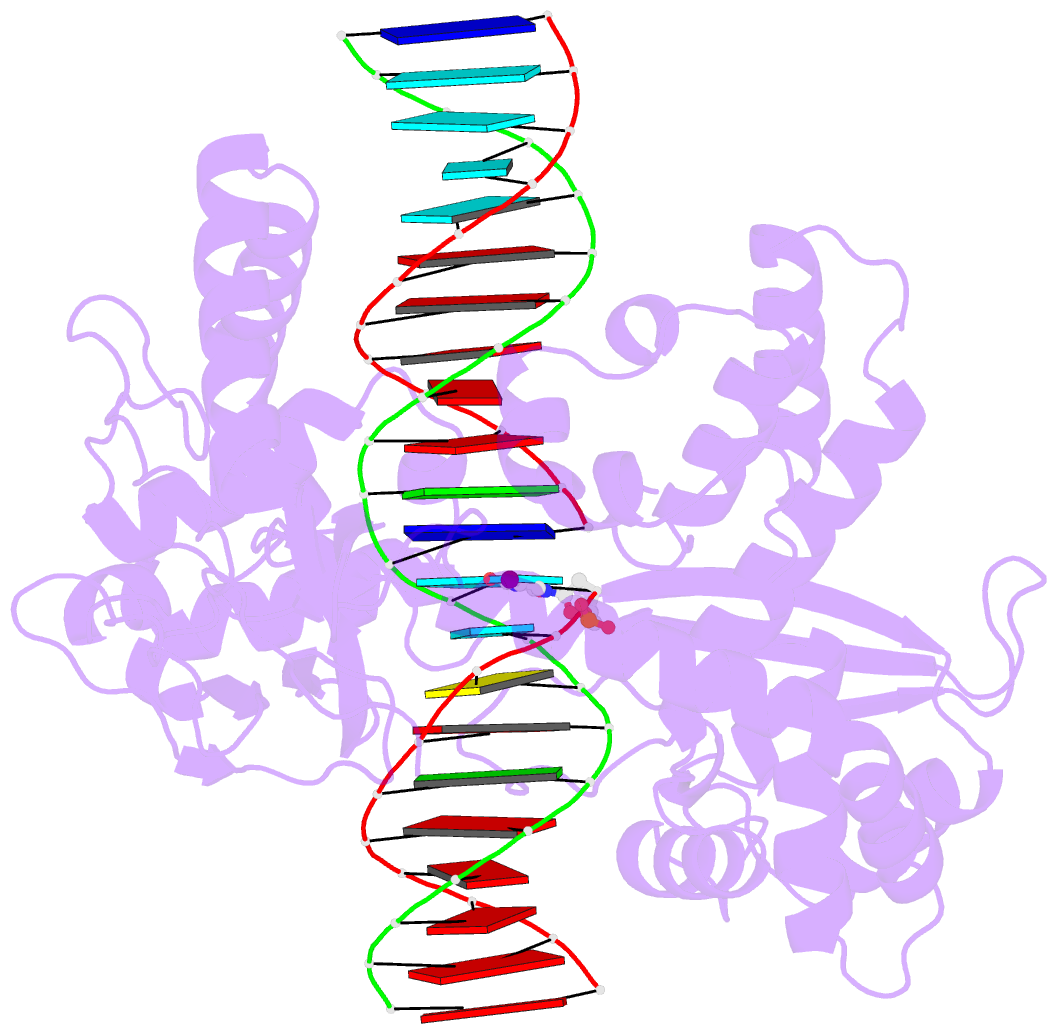
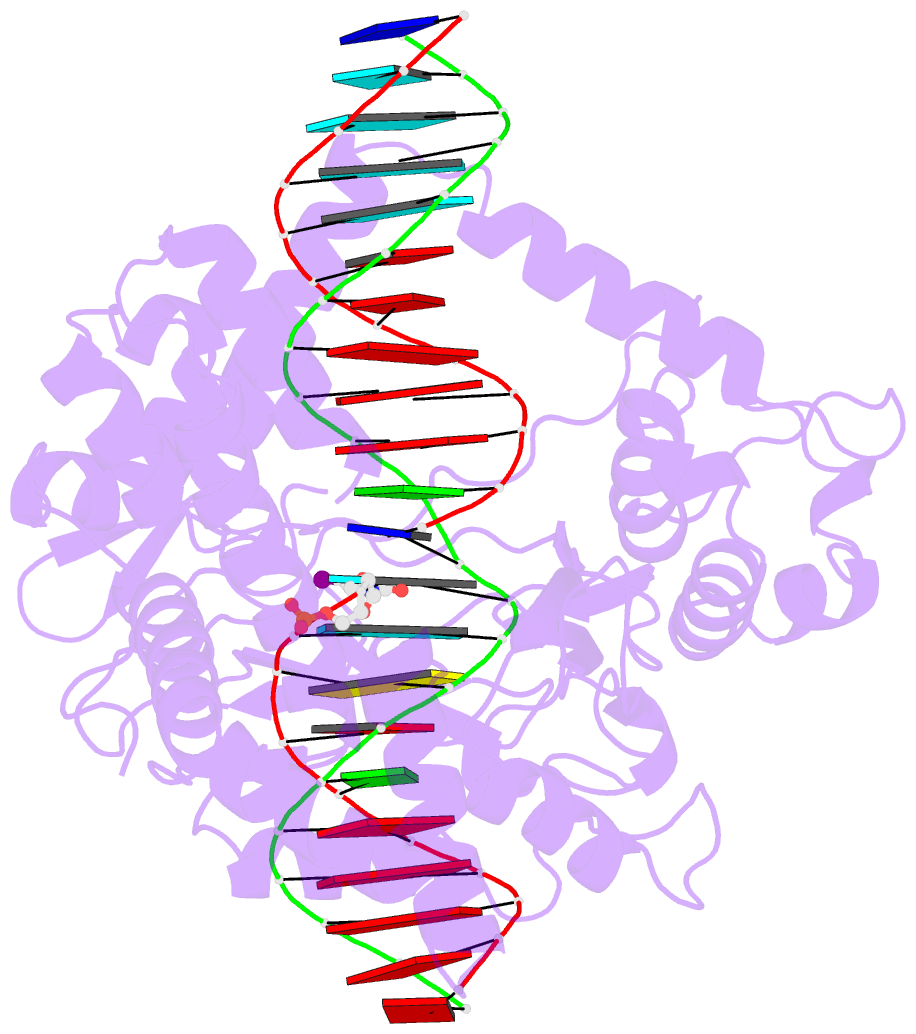
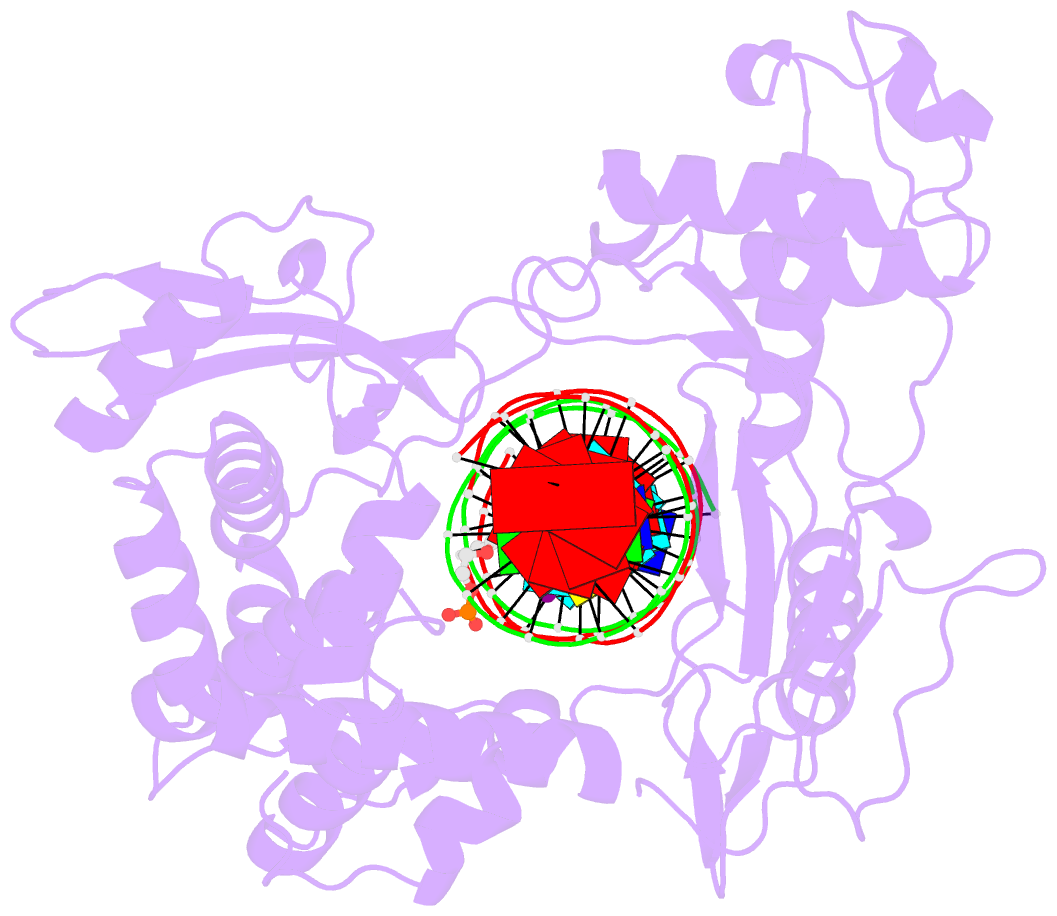
- Human reconstituted DNA topoisomerase i in covalent complex with a 22 base pair DNA duplex
- Reference
- Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG (1998): "Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA." Science, 279, 1504-1513. doi: 10.1126/science.279.5356.1504.
- Abstract
- Topoisomerases I promote the relaxation of DNA superhelical tension by introducing a transient single-stranded break in duplex DNA and are vital for the processes of replication, transcription, and recombination. The crystal structures at 2.1 and 2.5 angstrom resolution of reconstituted human topoisomerase I comprising the core and carboxyl-terminal domains in covalent and noncovalent complexes with 22-base pair DNA duplexes reveal an enzyme that "clamps" around essentially B-form DNA. The core domain and the first eight residues of the carboxyl-terminal domain of the enzyme, including the active-site nucleophile tyrosine-723, share significant structural similarity with the bacteriophage family of DNA integrases. A binding mode for the anticancer drug camptothecin is proposed on the basis of chemical and biochemical information combined with these three-dimensional structures of topoisomerase I-DNA complexes.