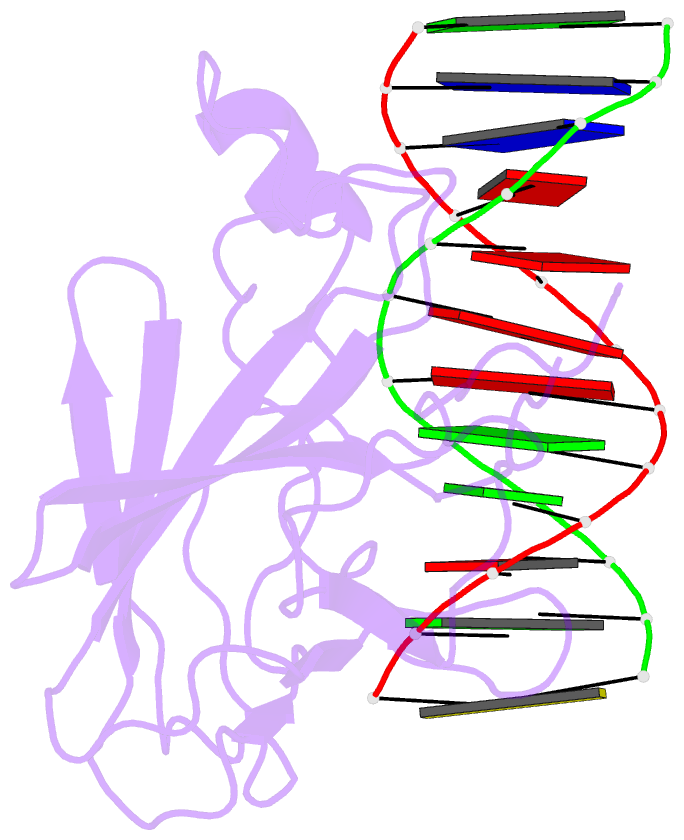
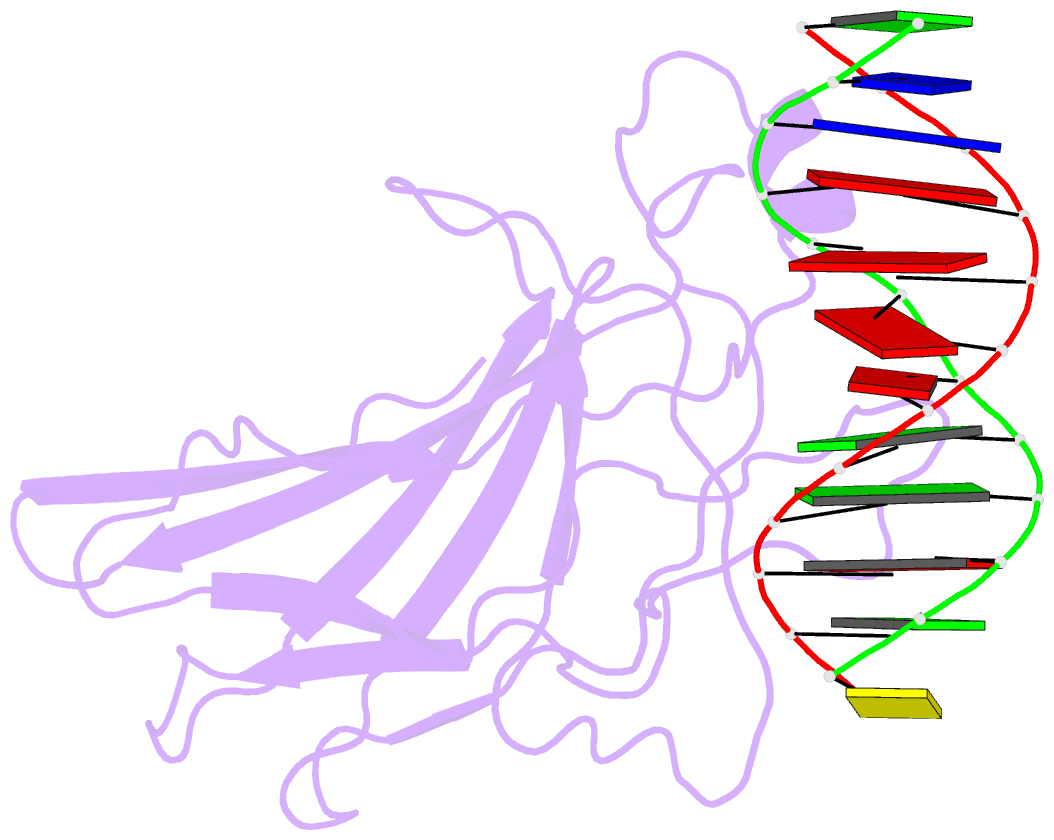
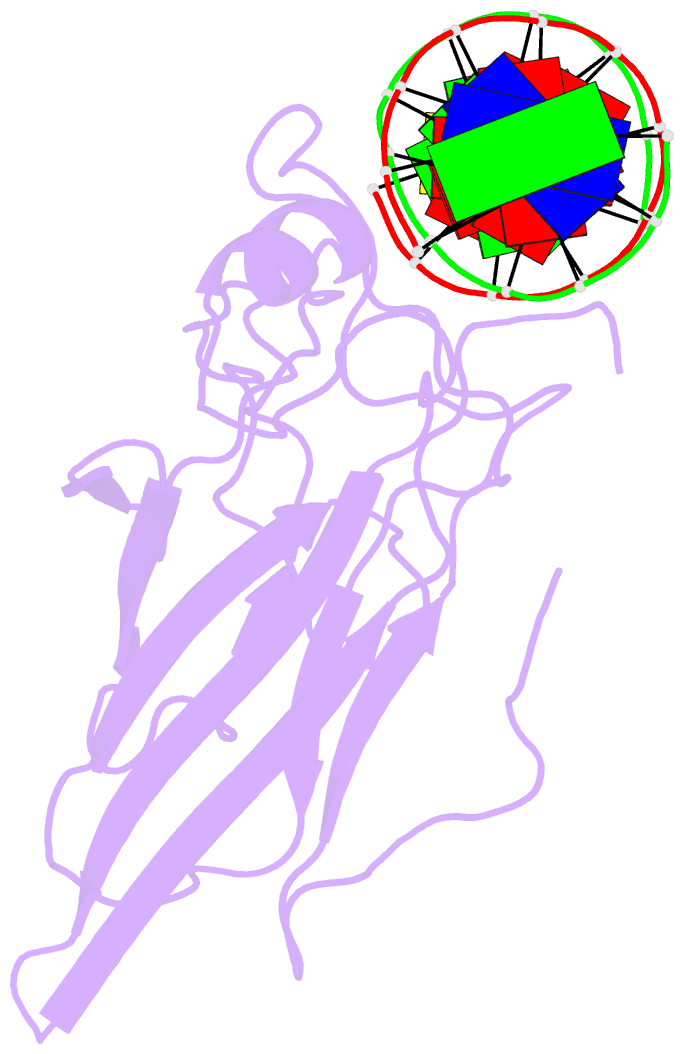
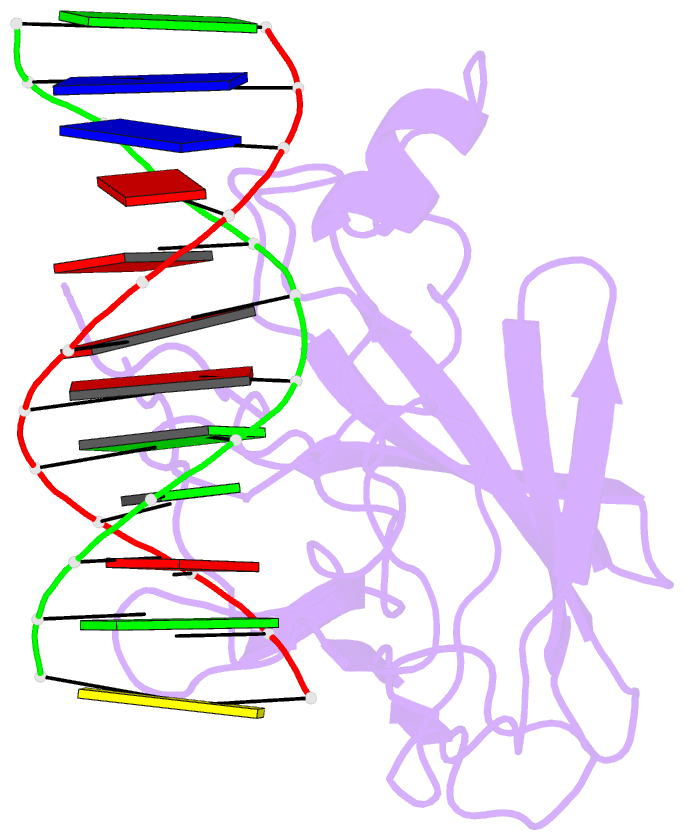
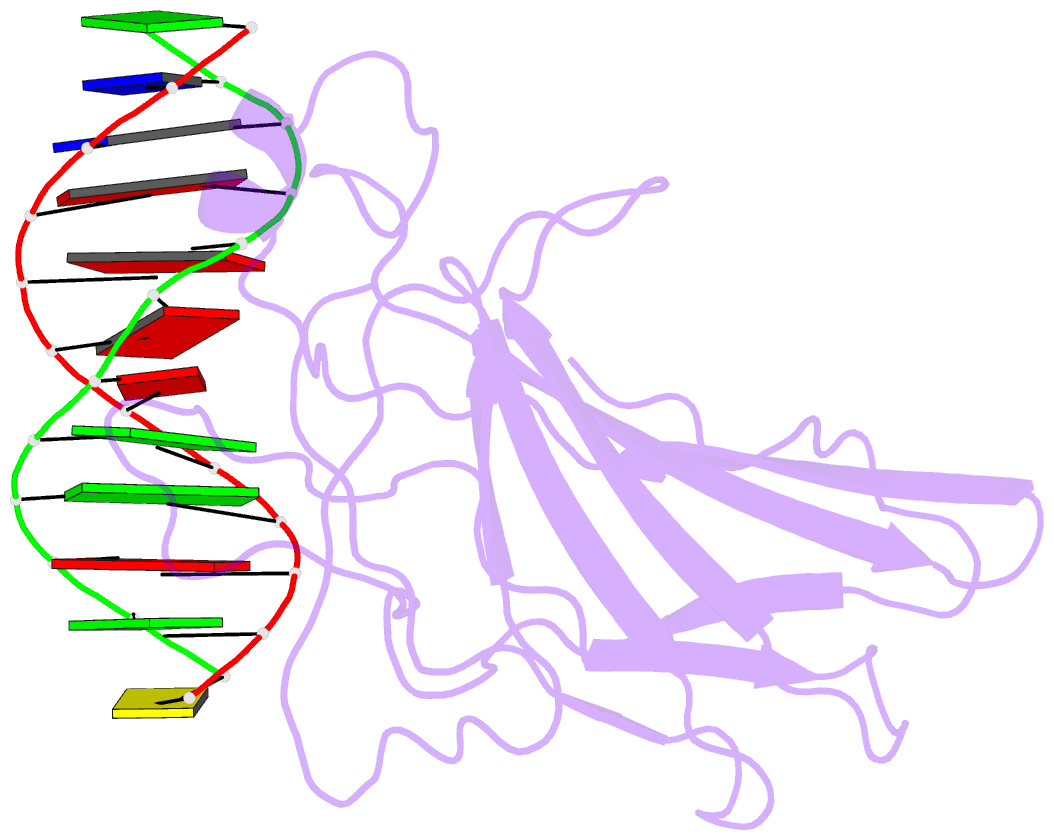
Summary information and primary citation
- PDB-id
- 1a66; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- NMR
- Summary
- Solution NMR structure of the core nfatc1-DNA complex, 18 structures
- Reference
- Zhou P, Sun LJ, Dotsch V, Wagner G, Verdine GL (1998): "Solution structure of the core NFATC1/DNA complex." Cell(Cambridge,Mass.), 92, 687-696. doi: 10.1016/S0092-8674(00)81136-8.
- Abstract
- The nuclear factor of the activated T cell (NFAT) family of transcription factors regulates cytokine gene expression by binding to the promoter/enhancer regions of antigen-responsive genes, usually in cooperation with heterologous DNA-binding partners. Here we report the solution structure of the binary complex formed between the core DNA-binding domain of human NFATC1 and the ARRE2 DNA site from the interleukin-2 promoter. The structure reveals that DNA binding induces the folding of key structural elements that are required for both sequence-specific recognition and the establishment of cooperative protein-protein contacts. The orientation of the NFAT DNA-binding domain observed in the binary NFATC1-DBD*/ DNA complex is distinct from that seen in the ternary NFATC2/AP-1/DNA complex, suggesting that the domain reorients upon formation of a cooperative transcriptional complex.