Summary information and primary citation
- PDB-id
- 1a6b; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- viral protein-DNA
- Method
- NMR
- Summary
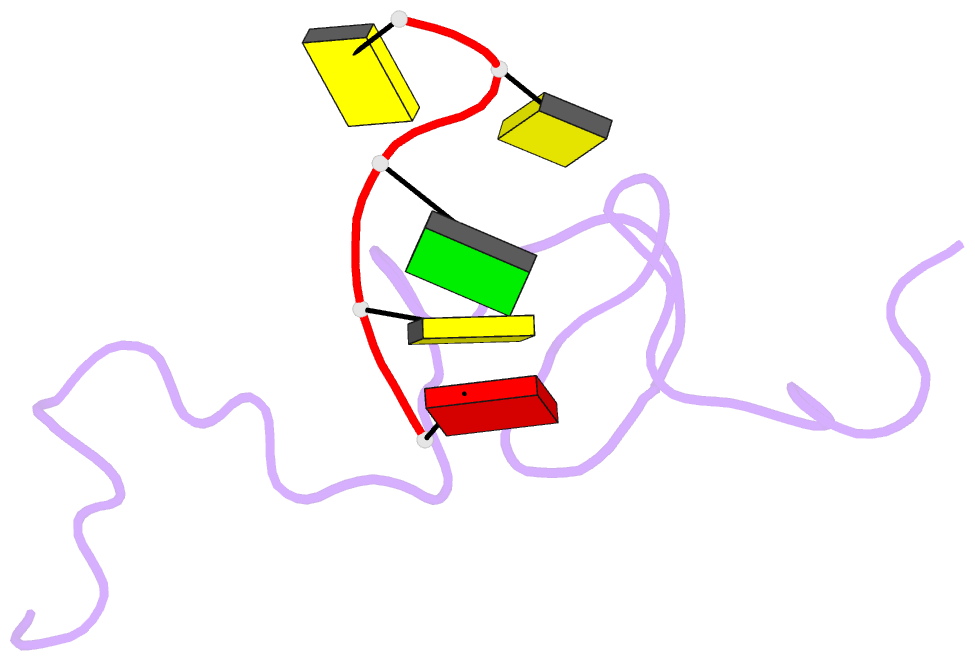
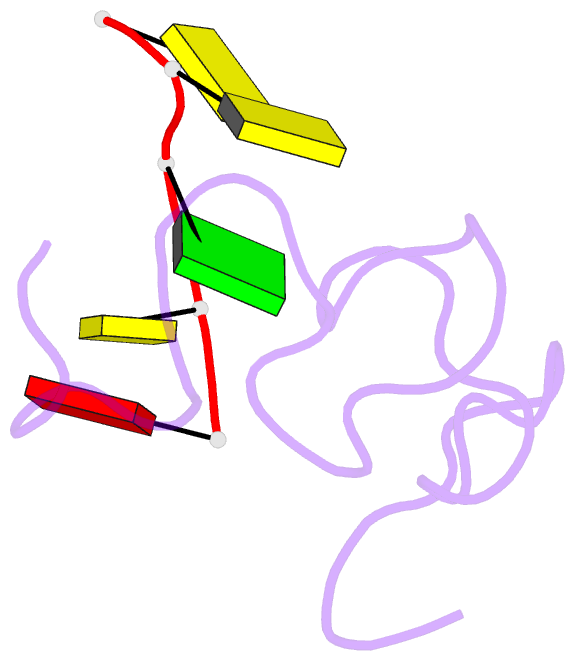
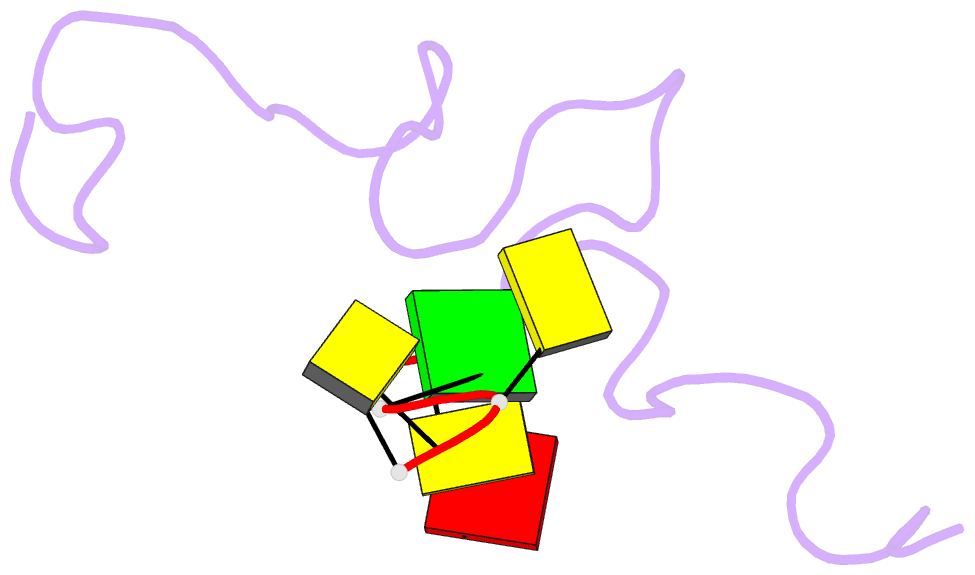
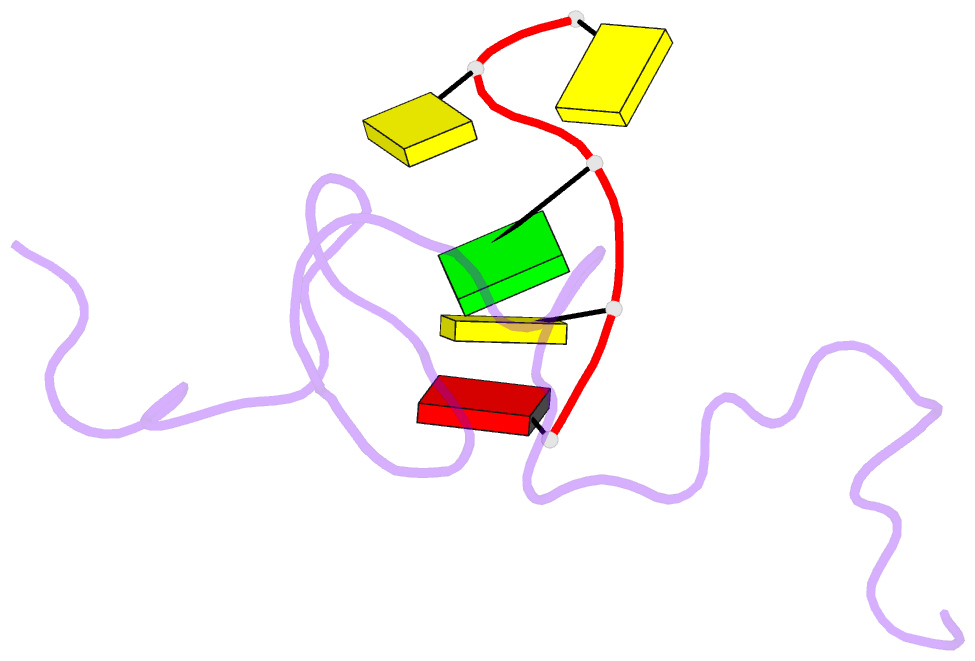
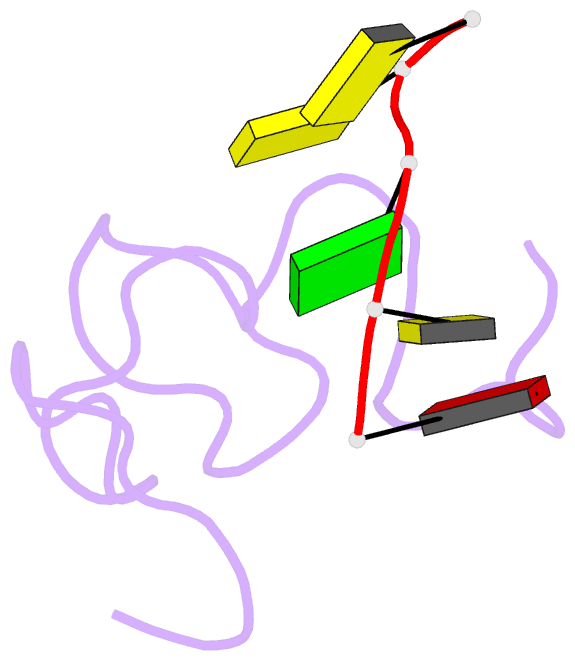
- NMR structure of the complex between the zinc finger protein ncp10 of moloney murine leukemia virus and a sequence of the psi-packaging domain of hiv-1, 20 structures
- Reference
- Schuler W, Dong C, Wecker K, Roques BP (1999): "NMR structure of the complex between the zinc finger protein NCp10 of Moloney murine leukemia virus and the single-stranded pentanucleotide d(ACGCC): comparison with HIV-NCp7 complexes." Biochemistry, 38, 12984-12994. doi: 10.1021/bi990378d.
- Abstract
- The structure of the 56 amino acid nucleocapsid protein NCp10 of retrovirus MoMuLV, which contains a single CX(2)CX(4)HX(4)C-type zinc finger, has been determined previously by NMR. The important role of NCp10 (or NCp7 for HIV-1) in the retroviral life cycle seems mainly related to their preferential binding to single-stranded nucleic acids. We report here the structure of the complex formed between the biologically active (14-53)NCp10 and the oligonucleotide d(ACGCC) in aqueous solution determined by 2D (1)H NMR based methods. The aromatic residue Trp(35) of NCp10 directs nucleic acid complexation as shown by its complete fluorescence quenching upon addition of d(ACGCC). (1)H and (31)P NMR studies support the insertion of Trp(35) between the G(3) and C(4) bases. A total of 577 NOE distance restraints, of which 40 were intermolecular, were used for the structure determination. The zinc finger provides a well-defined surface for the binding of d(ACGCC) through hydrophobic interactions and tryptophan stacking on the guanine. This latter interaction was also observed in the NMR-derived structures of the complexes between NCp7, which contains two successive zinc fingers, and single-stranded DNA and RNA, supporting the proposal for a major role played by aromatic residues of NCp proteins in nucleic acid recognition. Upon binding to the nucleotide a new loop in NCp10 that participates in the intermolecular interaction is formed. Additional interactions provided by positively charged residues surrounding the zinc finger appear necessary for tight binding. The structure of the complex NCp10-d(ACGCC) gives a structural explanation for the loss of virus infectivity following point mutations in the finger domain.