Summary information and primary citation
- PDB-id
- 1b01; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.56 Å)
- Summary
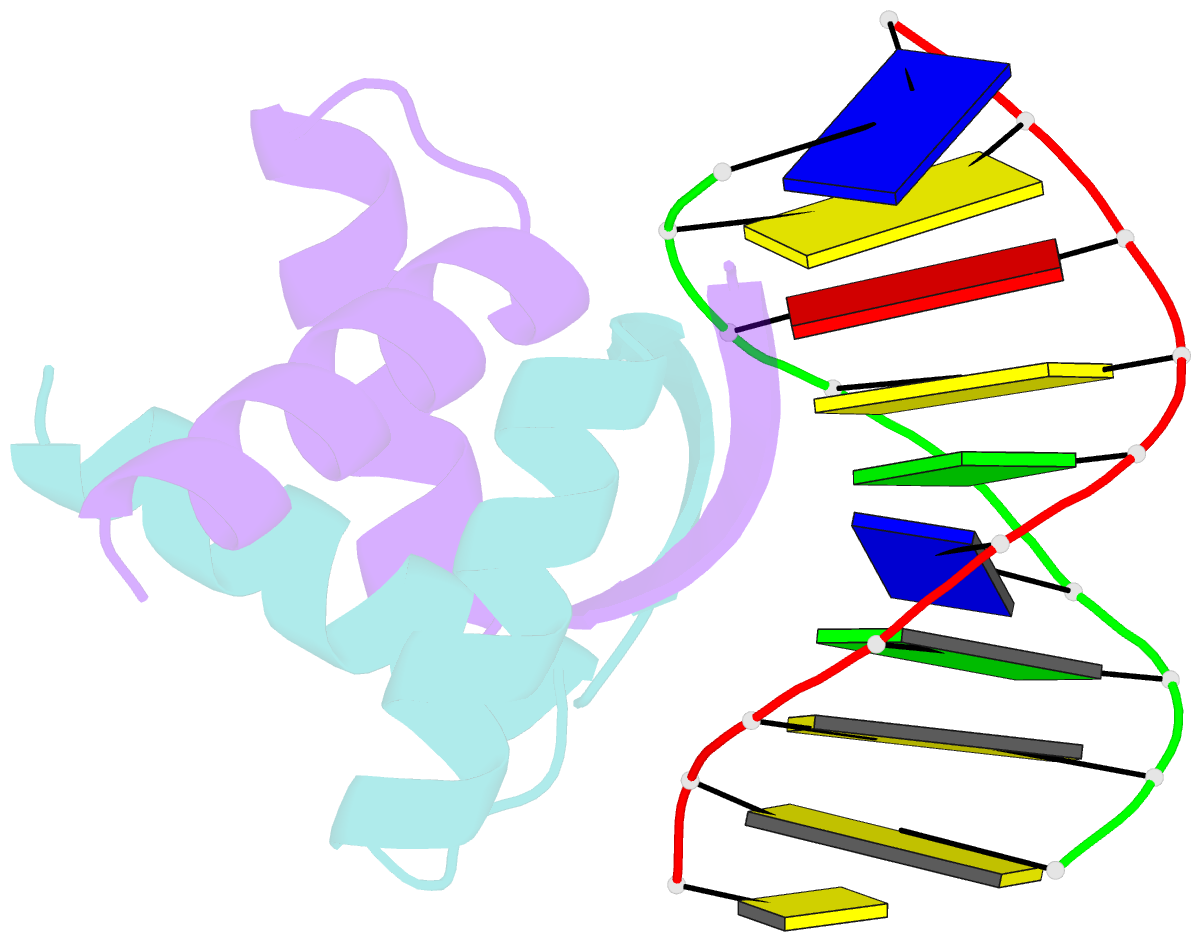
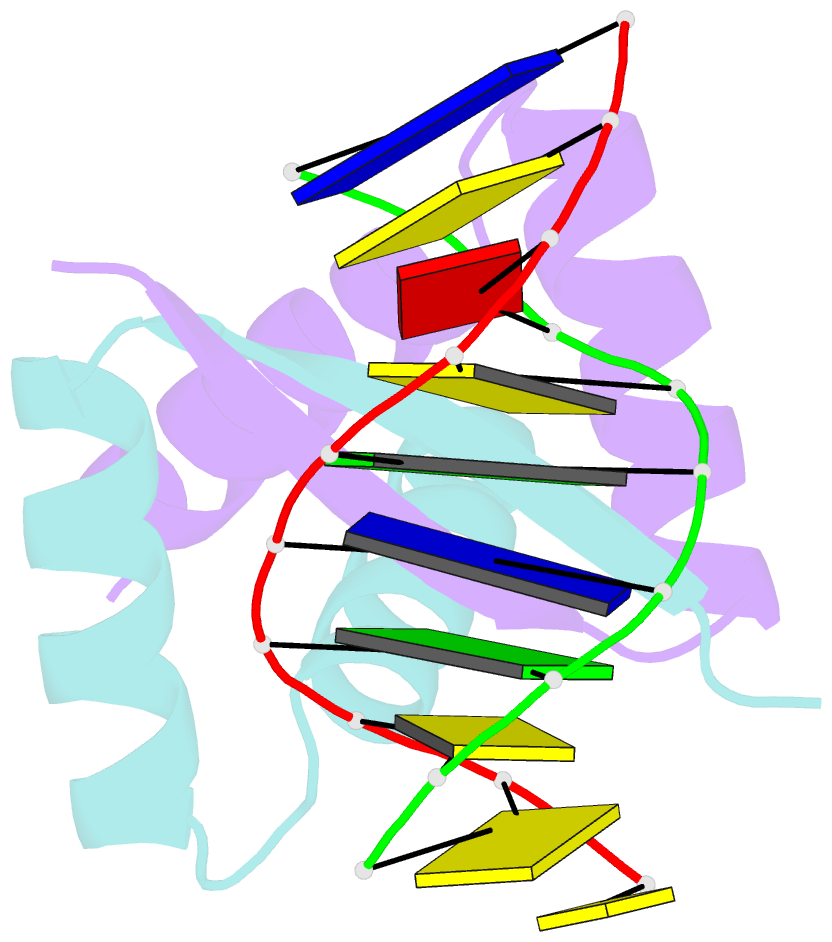
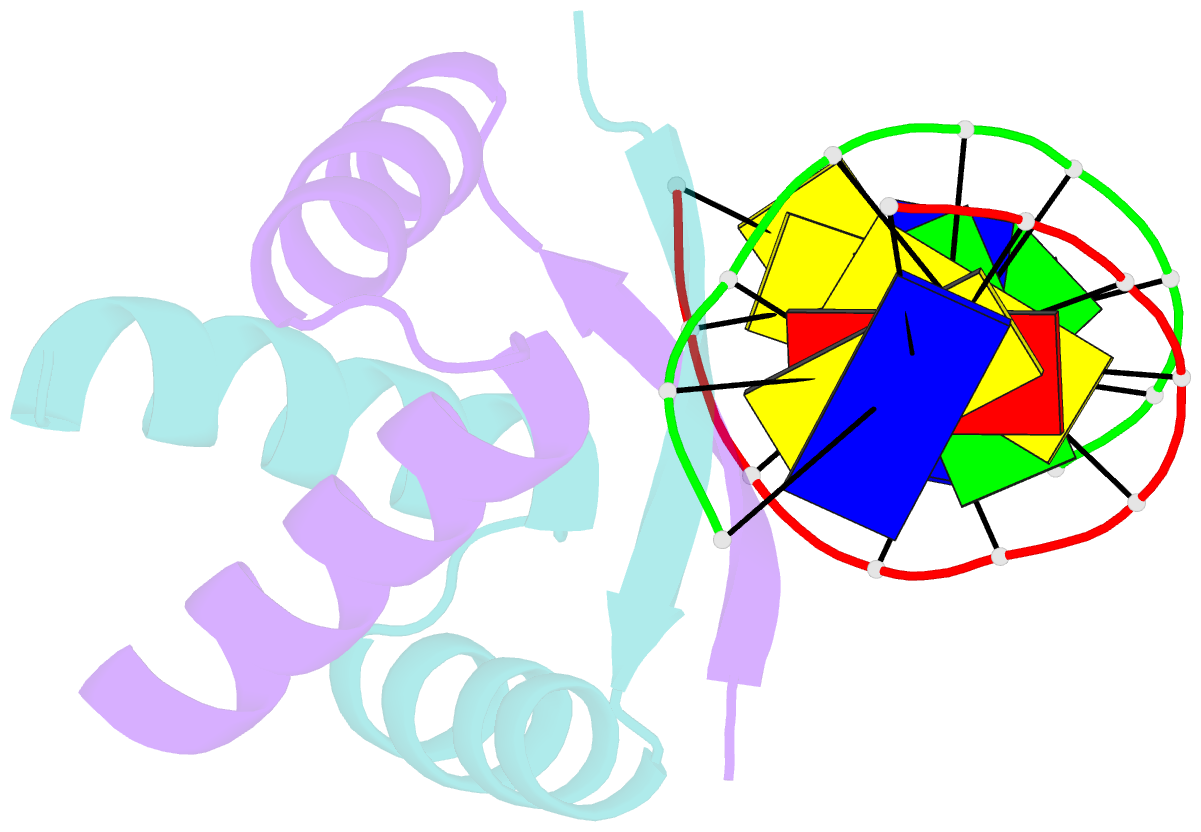
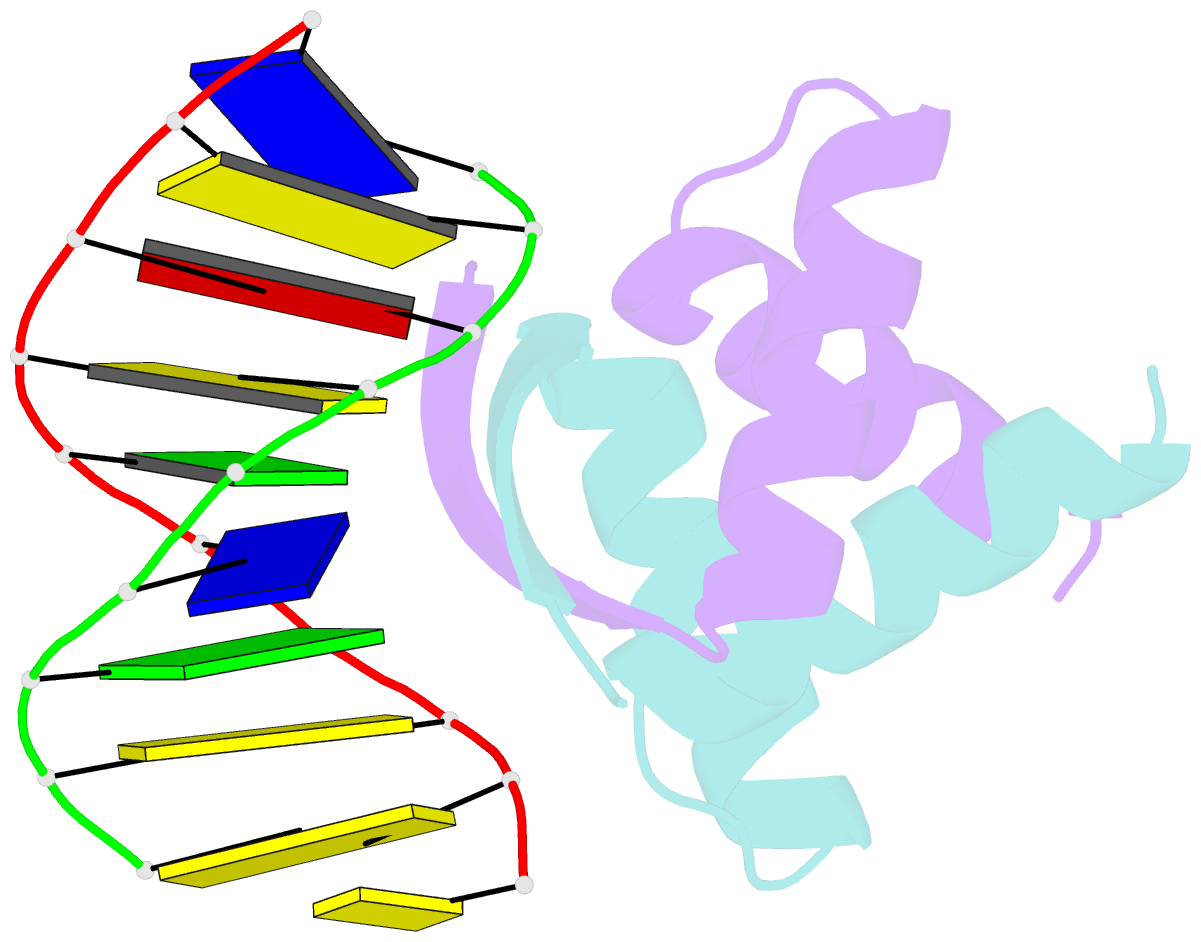
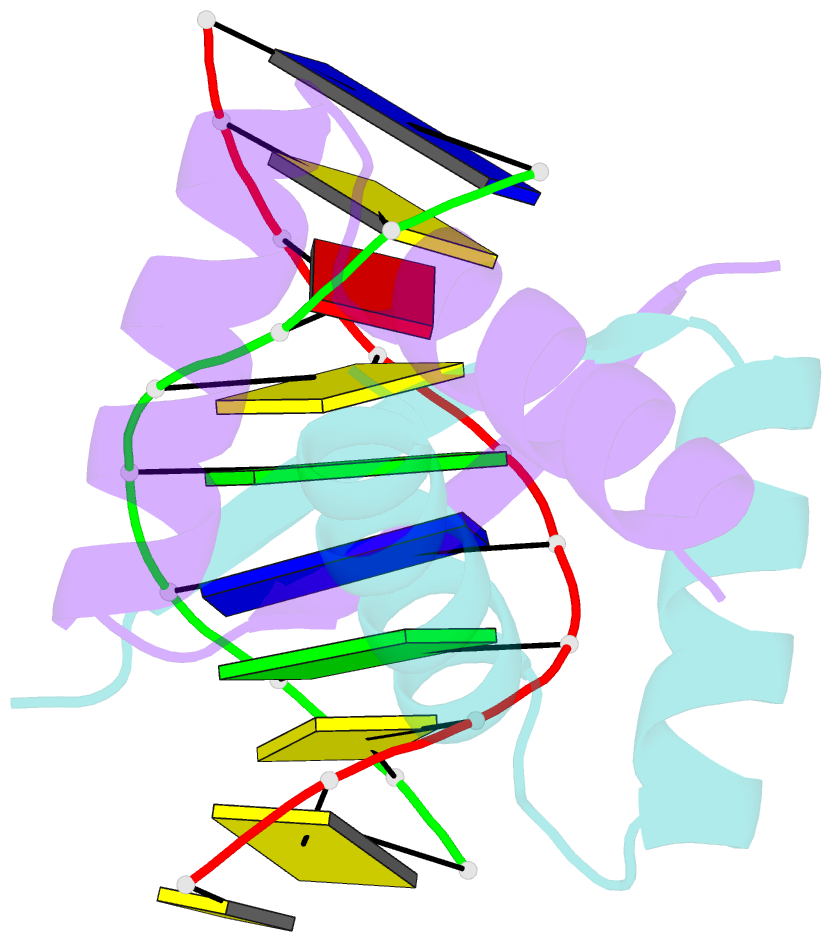
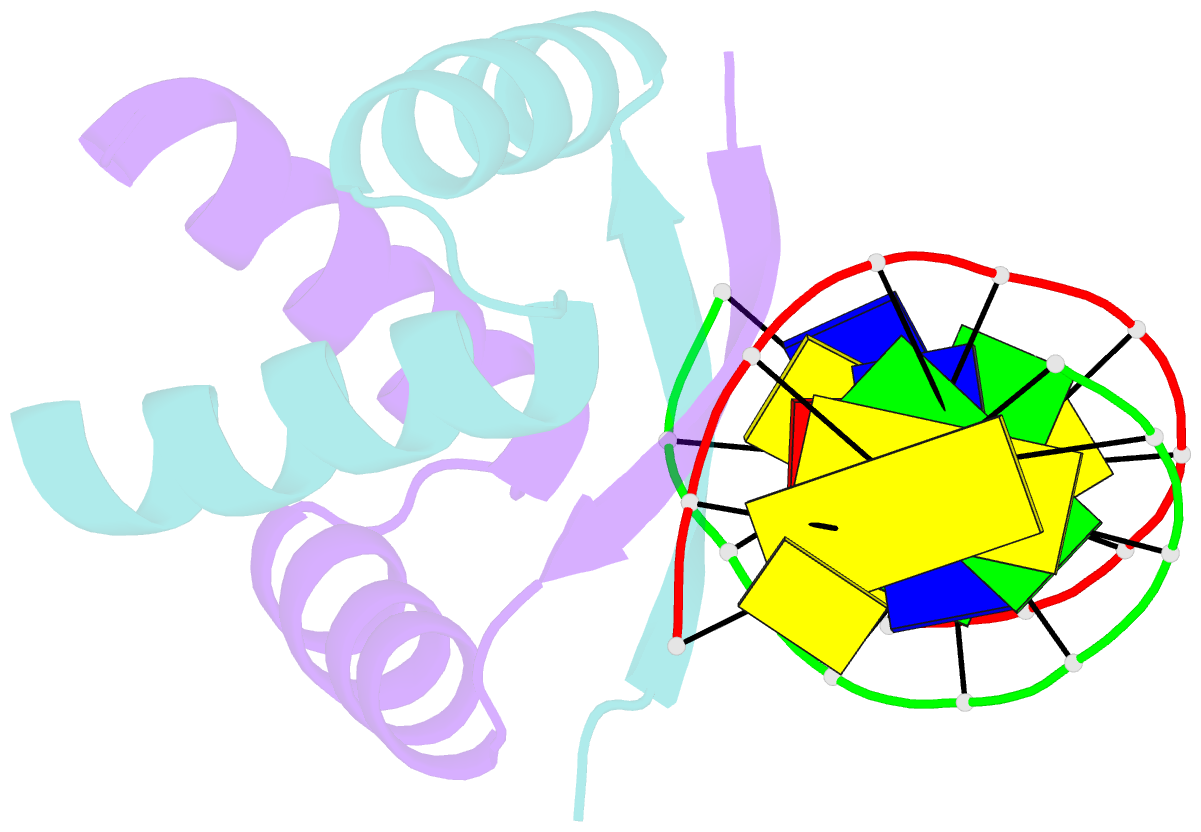
- Transcriptional repressor copg-DNA complex
- Reference
- Gomis-Ruth FX, Sola M, Acebo P, Parraga A, Guasch A, Eritja R, Gonzalez A, Espinosa M, del Solar G, Coll M (1998): "The structure of plasmid-encoded transcriptional repressor CopG unliganded and bound to its operator." EMBO J., 17, 7404-7415. doi: 10.1093/emboj/17.24.7404.
- Abstract
- The structure of the 45 amino acid transcriptional repressor, CopG, has been solved unliganded and bound to its target operator DNA. The protein, encoded by the promiscuous streptococcal plasmid pMV158, is involved in the control of plasmid copy number. The structure of this protein repressor, which is the shortest reported to date and the first isolated from a plasmid, has a homodimeric ribbon-helix-helix arrangement. It is the prototype for a family of homologous plasmid repressors. CopG cooperatively associates, completely protecting several turns on one face of the double helix in both directions from a 13-bp pseudosymmetric primary DNA recognition element. In the complex structure, one protein tetramer binds at one face of a 19-bp oligonucleotide, containing the pseudosymmetric element, with two beta-ribbons inserted into the major groove. The DNA is bent 60 degrees by compression of both major and minor grooves. The protein dimer displays topological similarity to Arc and MetJ repressors. Nevertheless, the functional tetramer has a unique structure with the two vicinal recognition ribbon elements at a short distance, thus inducing strong DNA bend. Further structural resemblance is found with helix-turn-helix regions of unrelated DNA-binding proteins. In contrast to these, however, the bihelical region of CopG has a role in oligomerization instead of DNA recognition. This observation unveils an evolutionary link between ribbon-helix-helix and helix-turn-helix proteins.