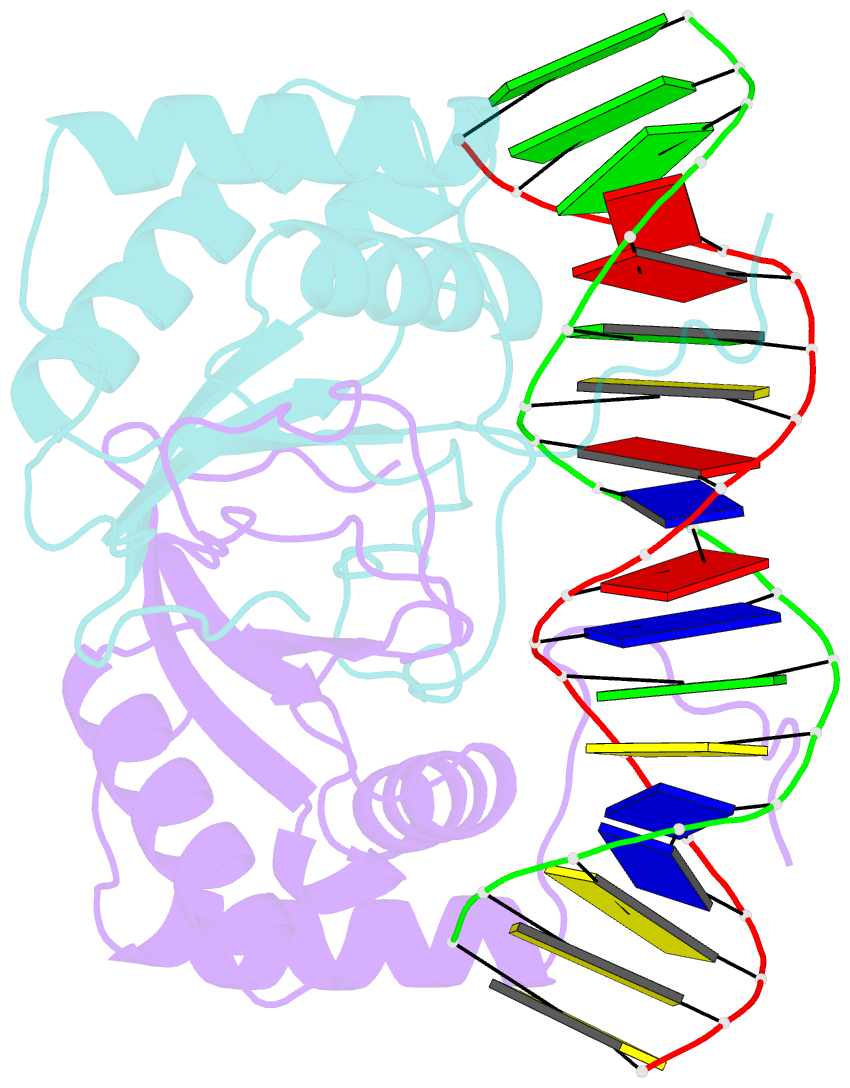
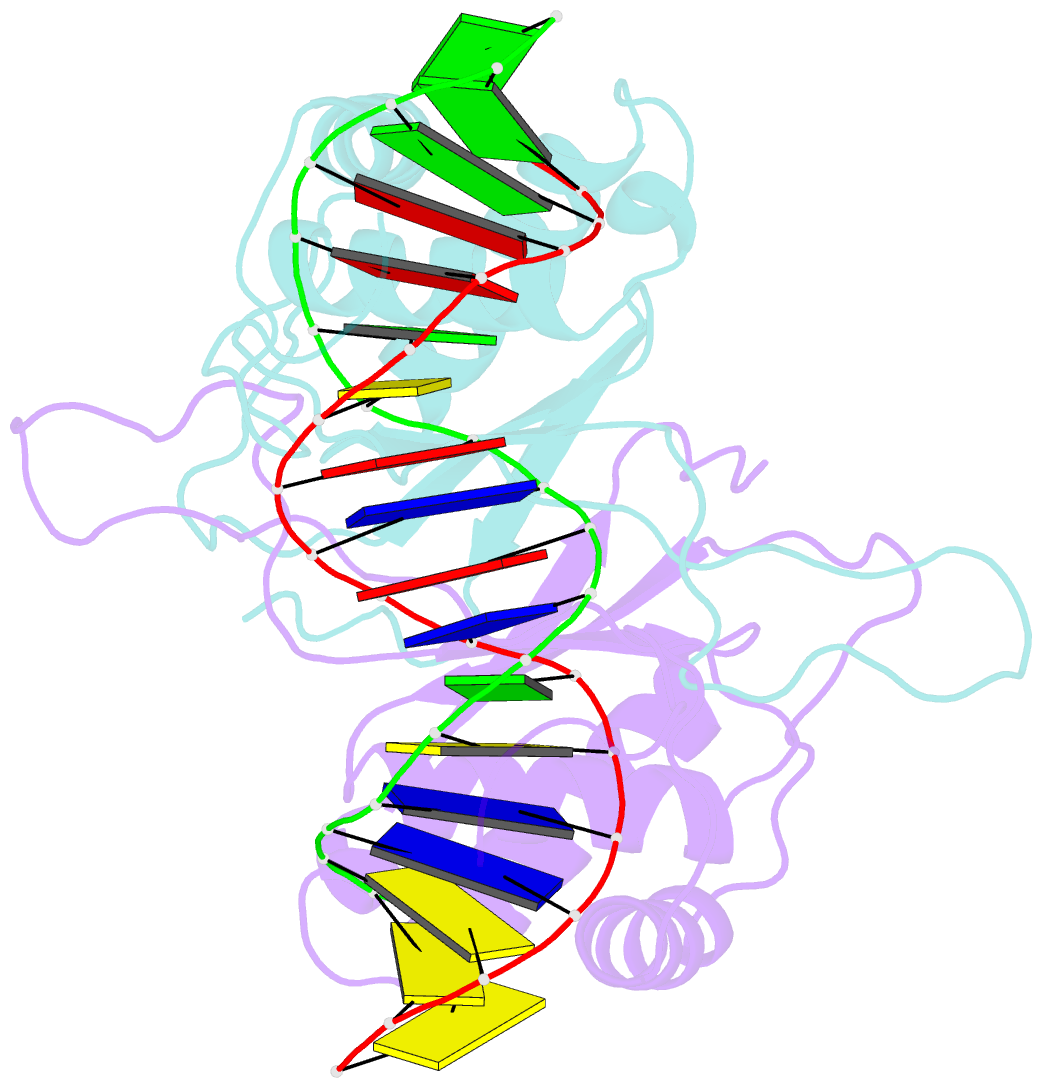
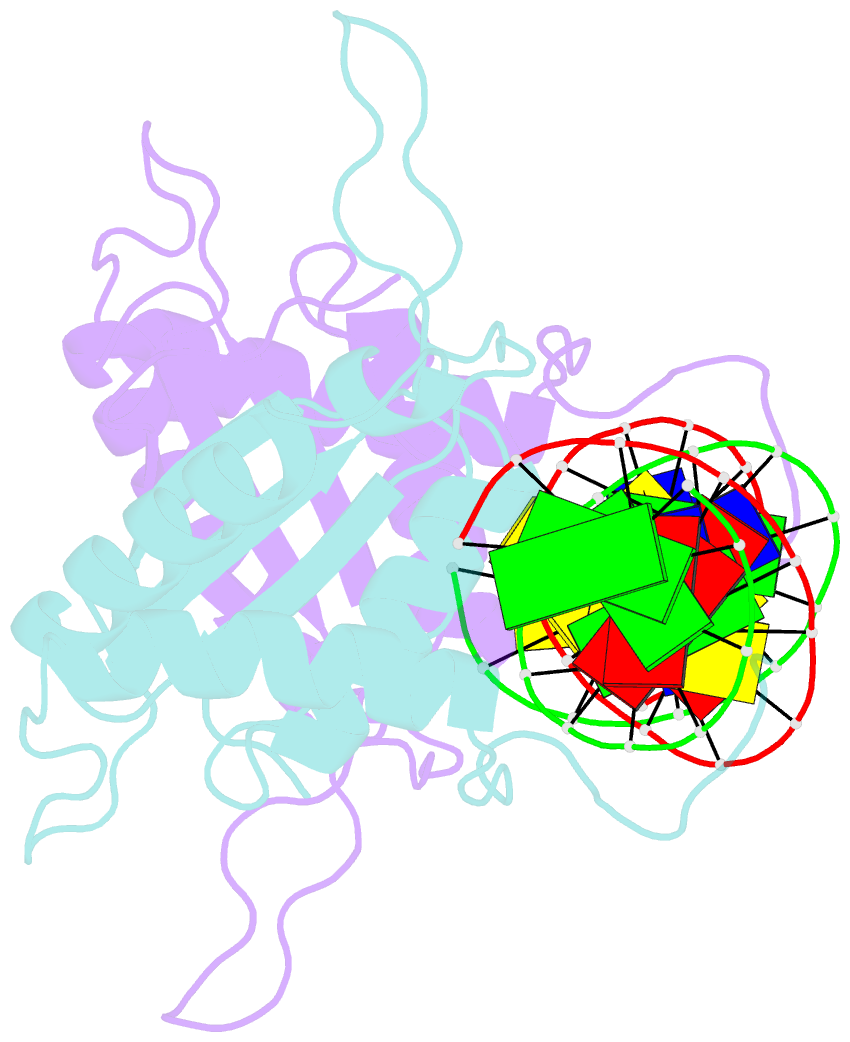
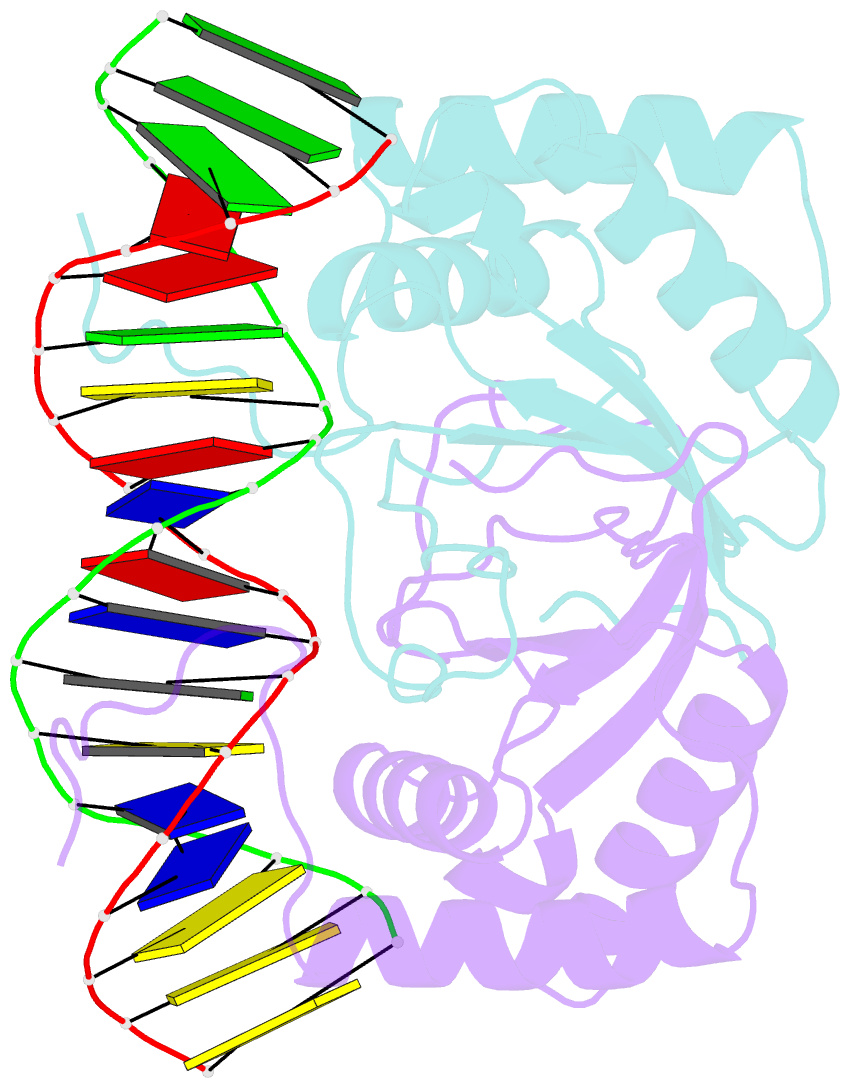
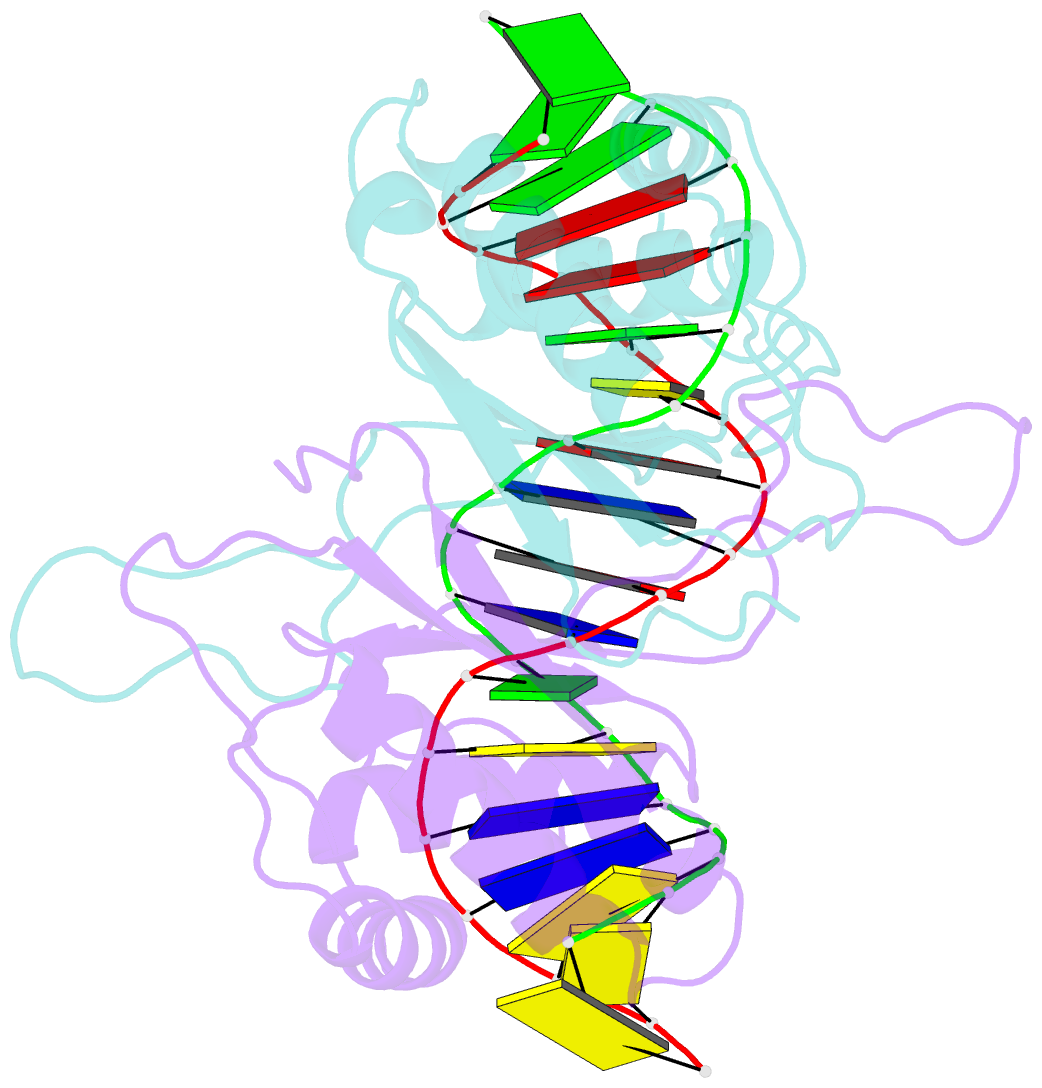
Summary information and primary citation
- PDB-id
- 1b3t; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- protein-DNA
- Method
- X-ray (2.2 Å)
- Summary
- Ebna-1 nuclear protein-DNA complex
- Reference
- Bochkarev A, Bochkareva E, Frappier L, Edwards AM (1998): "The 2.2 A structure of a permanganate-sensitive DNA site bound by the Epstein-Barr virus origin binding protein, EBNA1." J.Mol.Biol., 284, 1273-1278. doi: 10.1006/jmbi.1998.2247.
- Abstract
- Epstein-Barr nuclear antigen 1 (EBNA1) binds to four recognition sites in the minimal origin of latent DNA replication of Epstein-Barr virus and activates latent-phase replication of the viral genomes. Two of these EBNA1 binding sites become sensitive to permanganate oxidation when bound by the DNA binding and dimerization domains of EBNA1. We have previously solved the co-crystal structure of this EBNA1 fragment bound to a consensus recognition site that is not sensitive to permanganate oxidation (CS). To understand the structural difference that underlies the permanganate sensitivity of EBNA1 binding sites, we have now solved the crystal structure of the EBNA1 DNA-binding and dimerization domains bound to a permanganate-sensitive site (CSA/T). Comparisons of permanganate-sensitive and insensitive EBNA1-DNA complexes have revealed only minor differences in protein and DNA structures. In the EBNA1-CSA/T structure, interstrand H-bonds for three consecutive base-pairs centered over the permanganate-sensitive thymine base are lengthened relative to the corresponding bonds in the EBNA1-CS complex, and three potential intrastrand H-bonds were observed between adjacent bases. We also observed that both the CS and CSA/T sequences are overwound by EBNA1 in the vicinity of the permanganate-sensitive thymine base. Finally, we show that the permanganate-sensitive thymine base in the CSA/T-EBNA1 complex is more accessible to solvent than the corresponding T in the EBNA-CS complex.