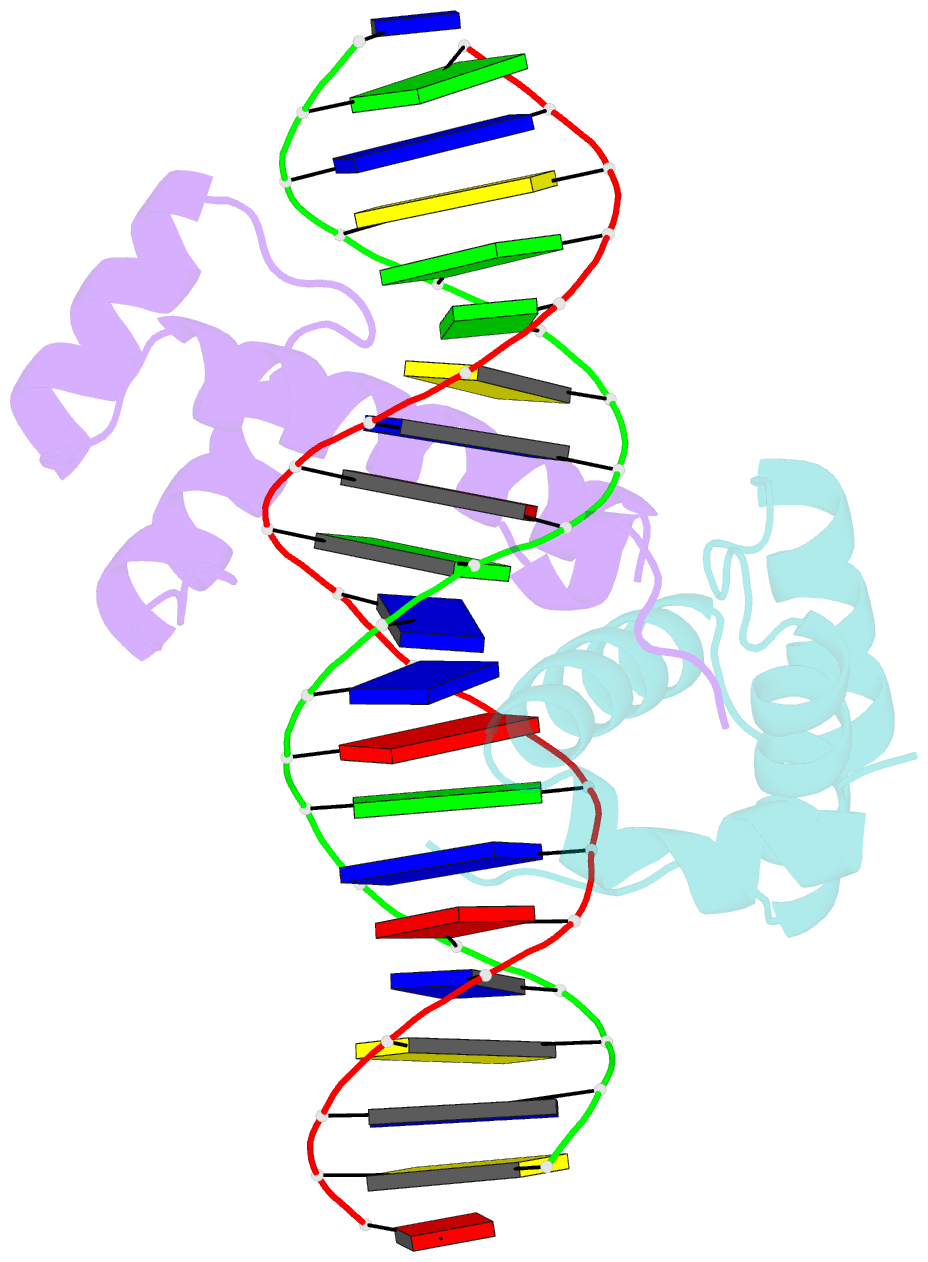
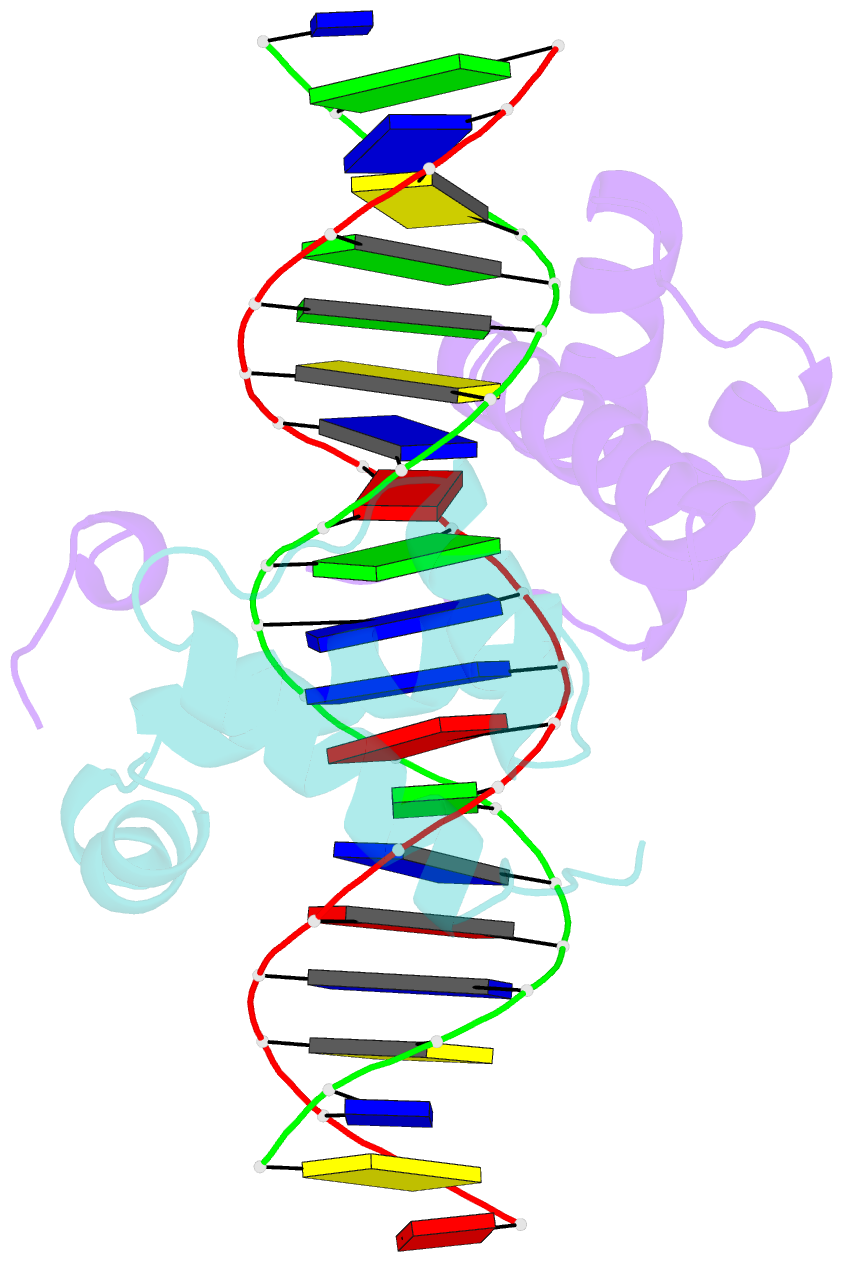
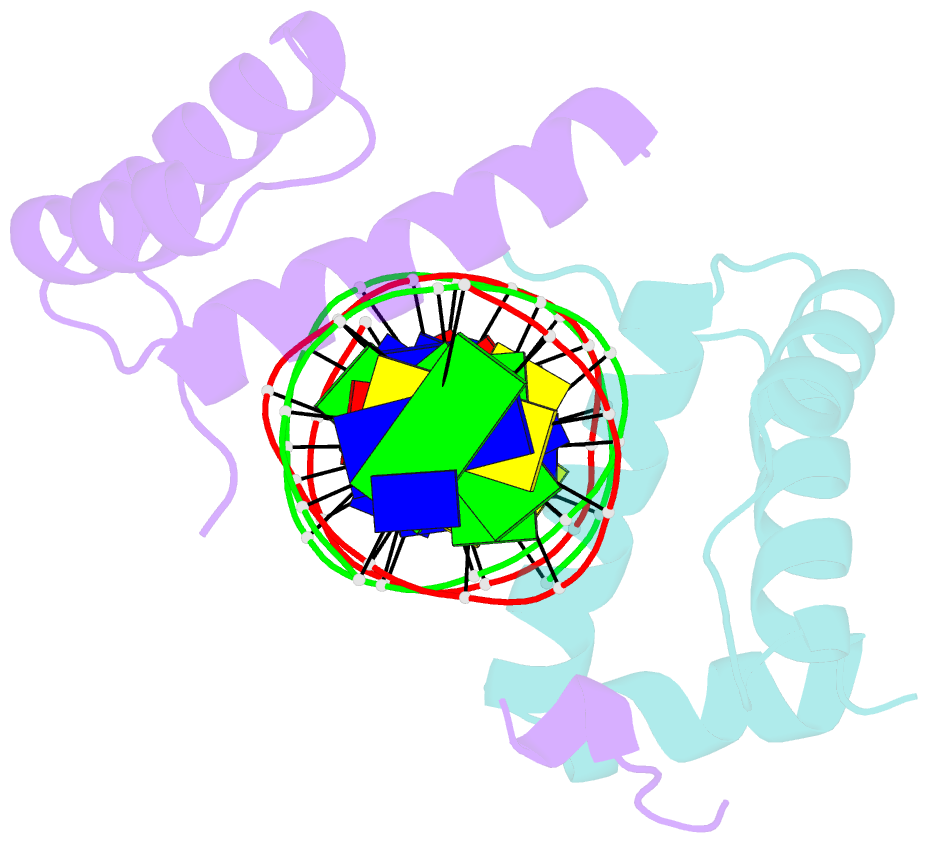
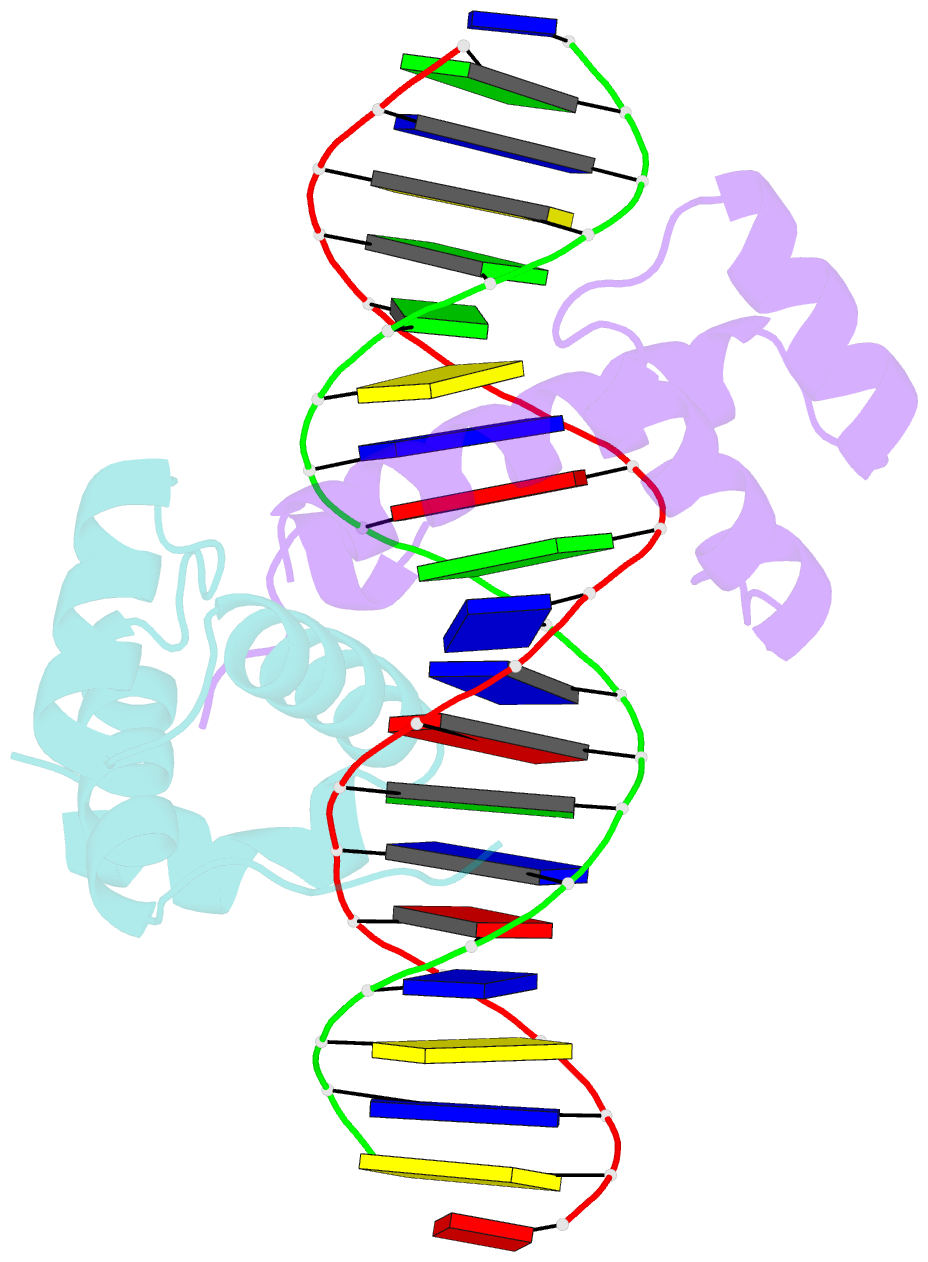
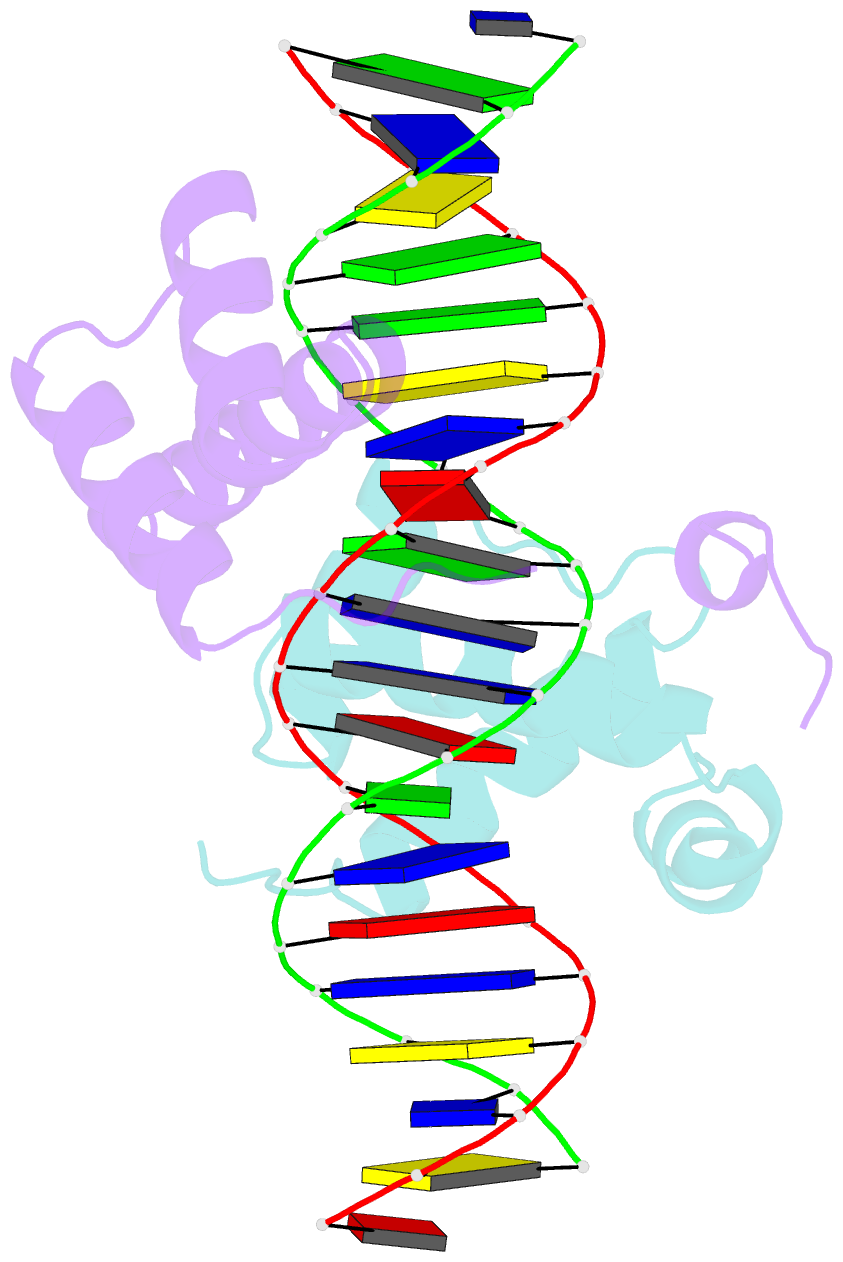
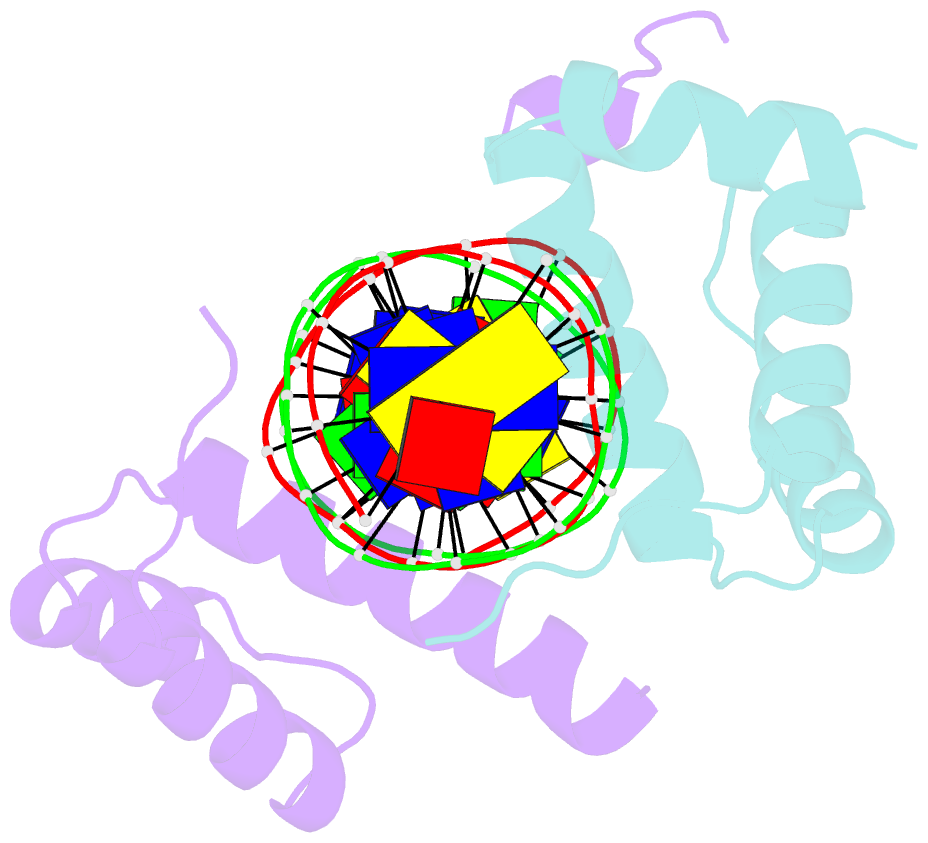
Summary information and primary citation
- PDB-id
- 1b72; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- protein-DNA
- Method
- X-ray (2.35 Å)
- Summary
- Pbx1, homeobox protein hox-b1-DNA ternary complex
- Reference
- Piper DE, Batchelor AH, Chang CP, Cleary ML, Wolberger C (1999): "Structure of a HoxB1-Pbx1 heterodimer bound to DNA: role of the hexapeptide and a fourth homeodomain helix in complex formation." Cell(Cambridge,Mass.), 96, 587-597. doi: 10.1016/S0092-8674(00)80662-5.
- Abstract
- Hox homeodomain proteins are developmental regulators that determine body plan in a variety of organisms. A majority of the vertebrate Hox proteins bind DNA as heterodimers with the Pbx1 homeodomain protein. We report here the 2.35 A structure of a ternary complex containing a human HoxB1-Pbx1 heterodimer bound to DNA. Heterodimer contacts are mediated by the hexapeptide of HoxB1, which binds in a pocket in the Pbx1 protein formed in part by a three-amino acid insertion in the Pbx1 homeodomain. The Pbx1 DNA-binding domain is larger than the canonical homeodomain, containing an additional alpha helix that appears to contribute to binding of the HoxB1 hexapeptide and to stable binding of Pbx1 to DNA. The structure suggests a model for modulation of Hox DNA binding activity by Pbx1 and related proteins.