Summary information and primary citation
- PDB-id
- 1bbx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- NMR
- Summary
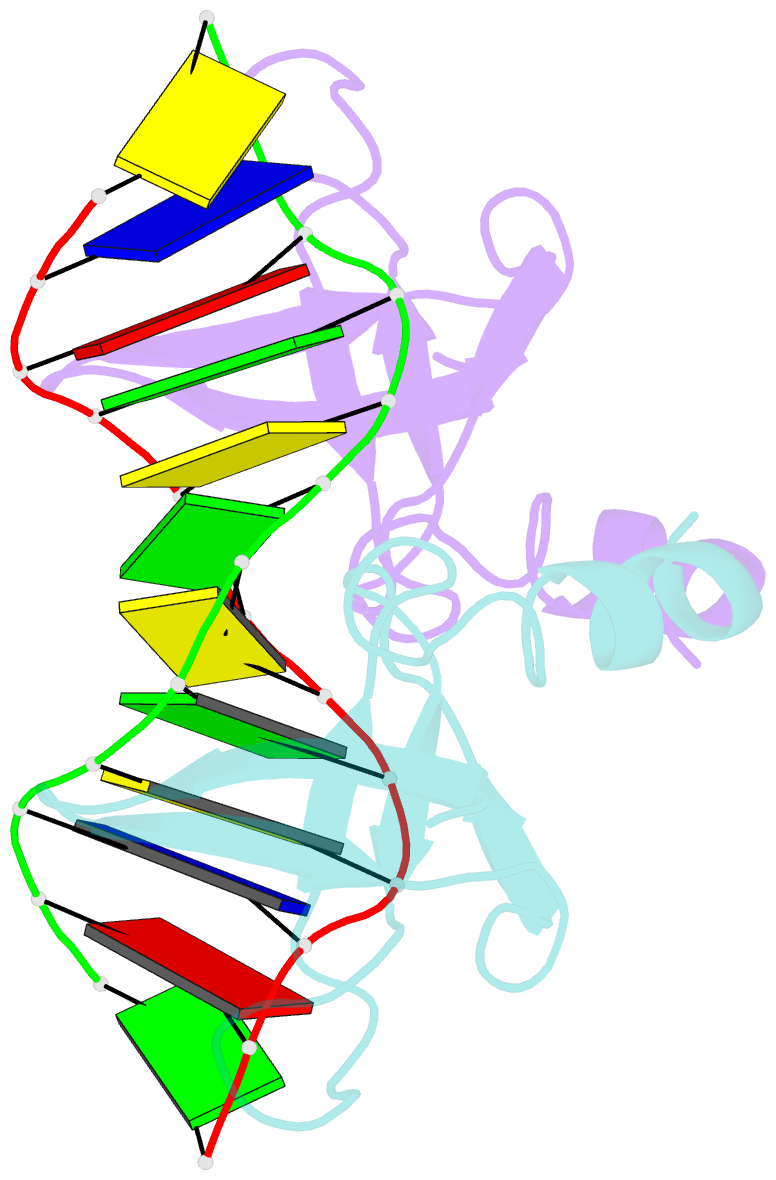
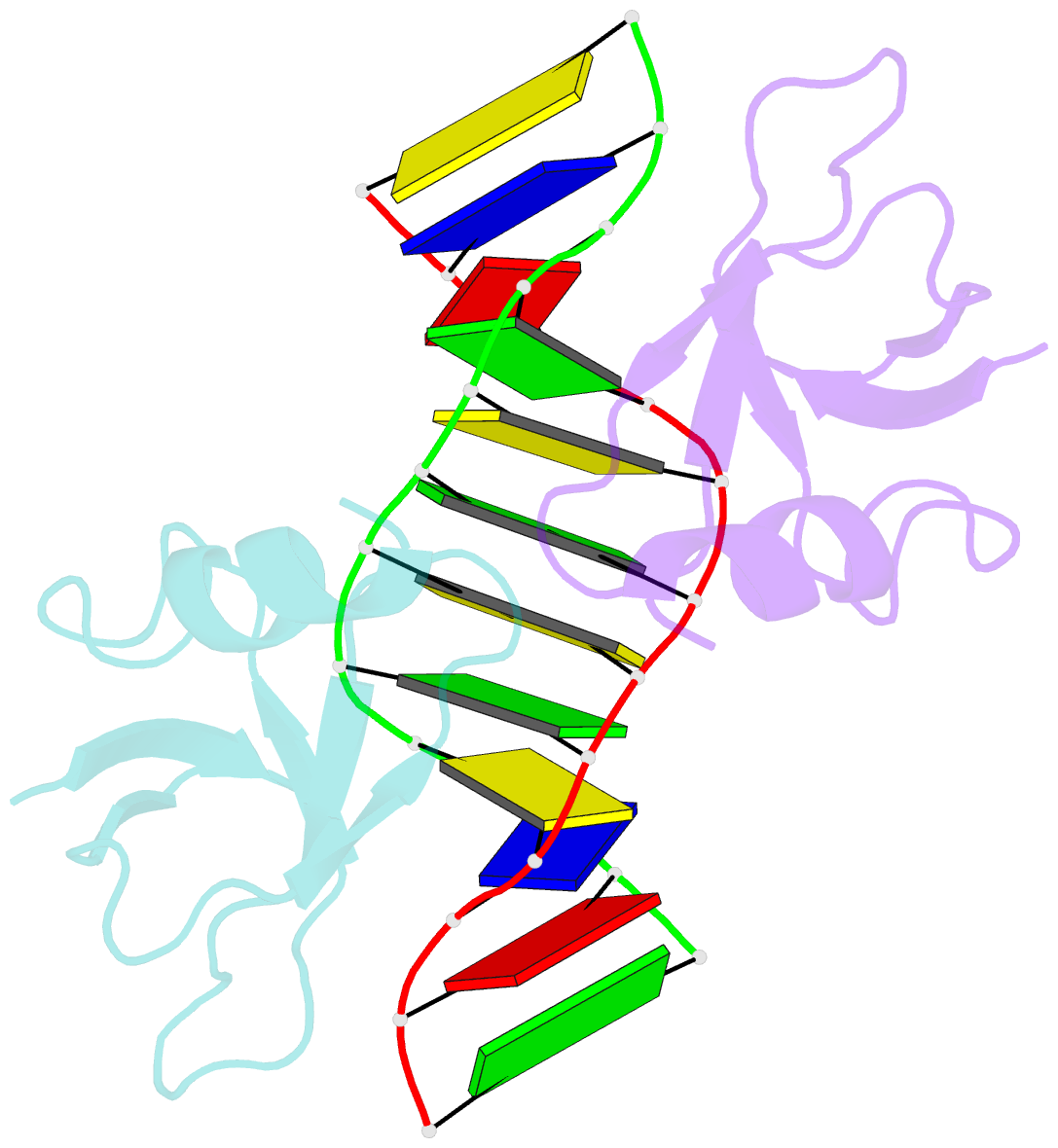
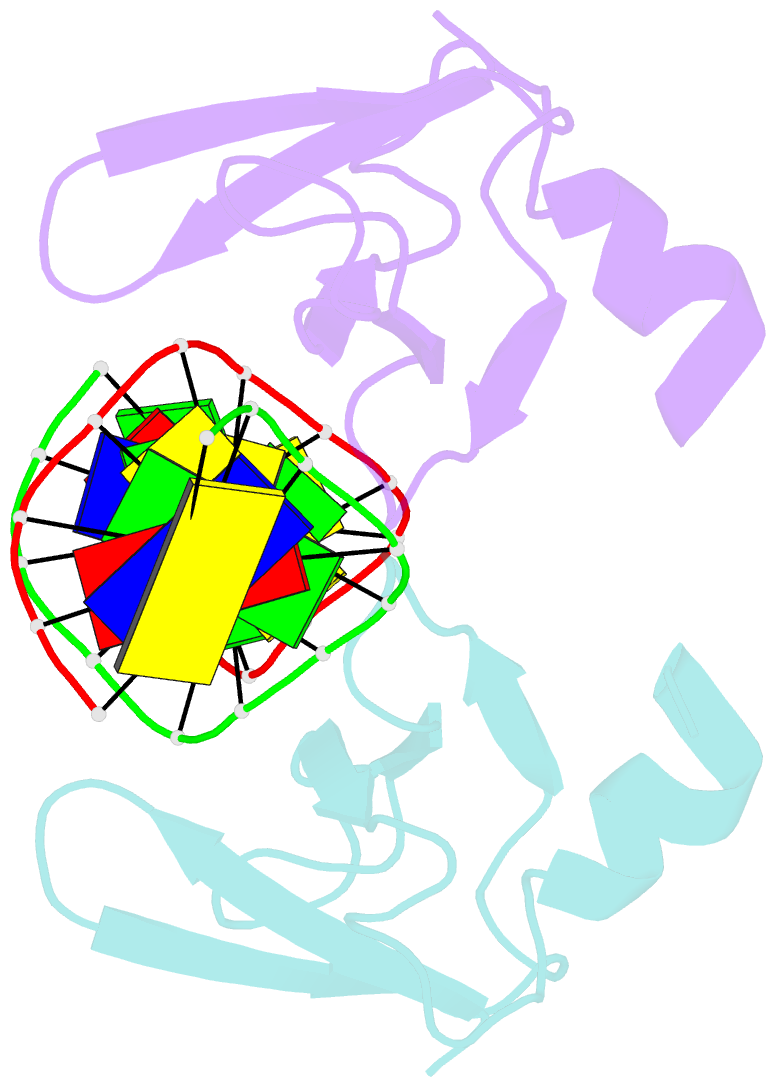
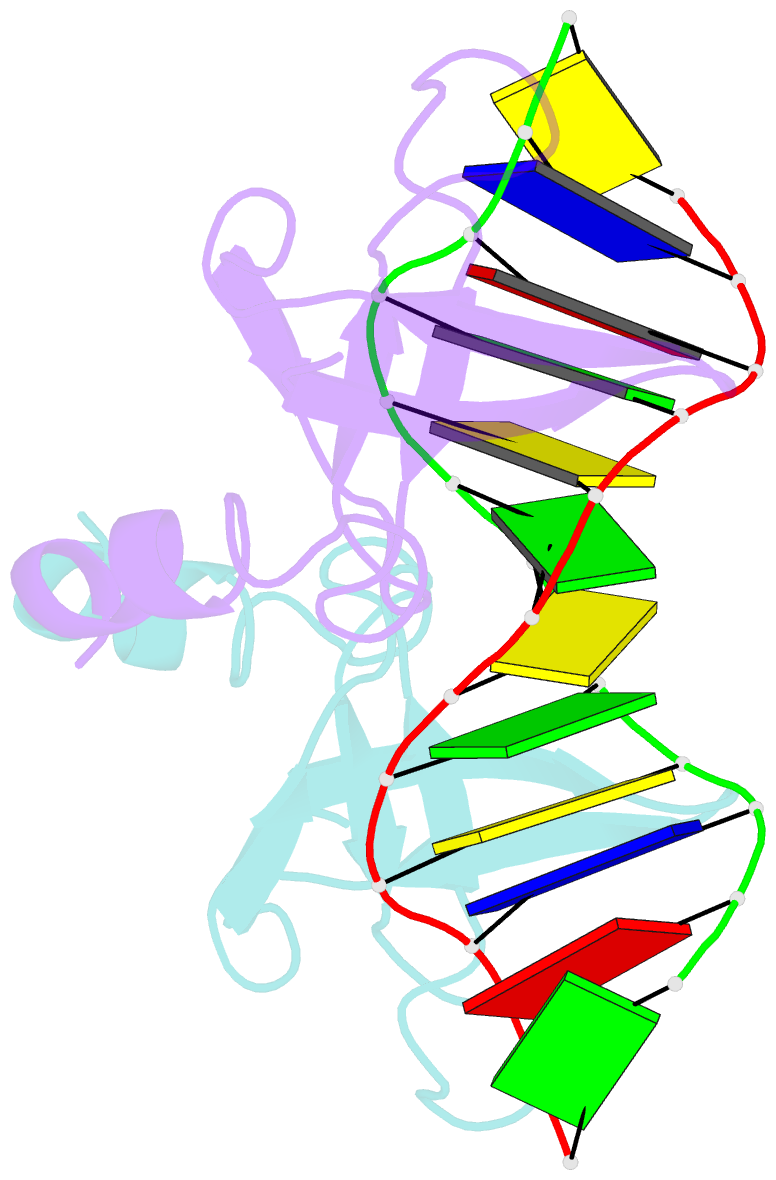
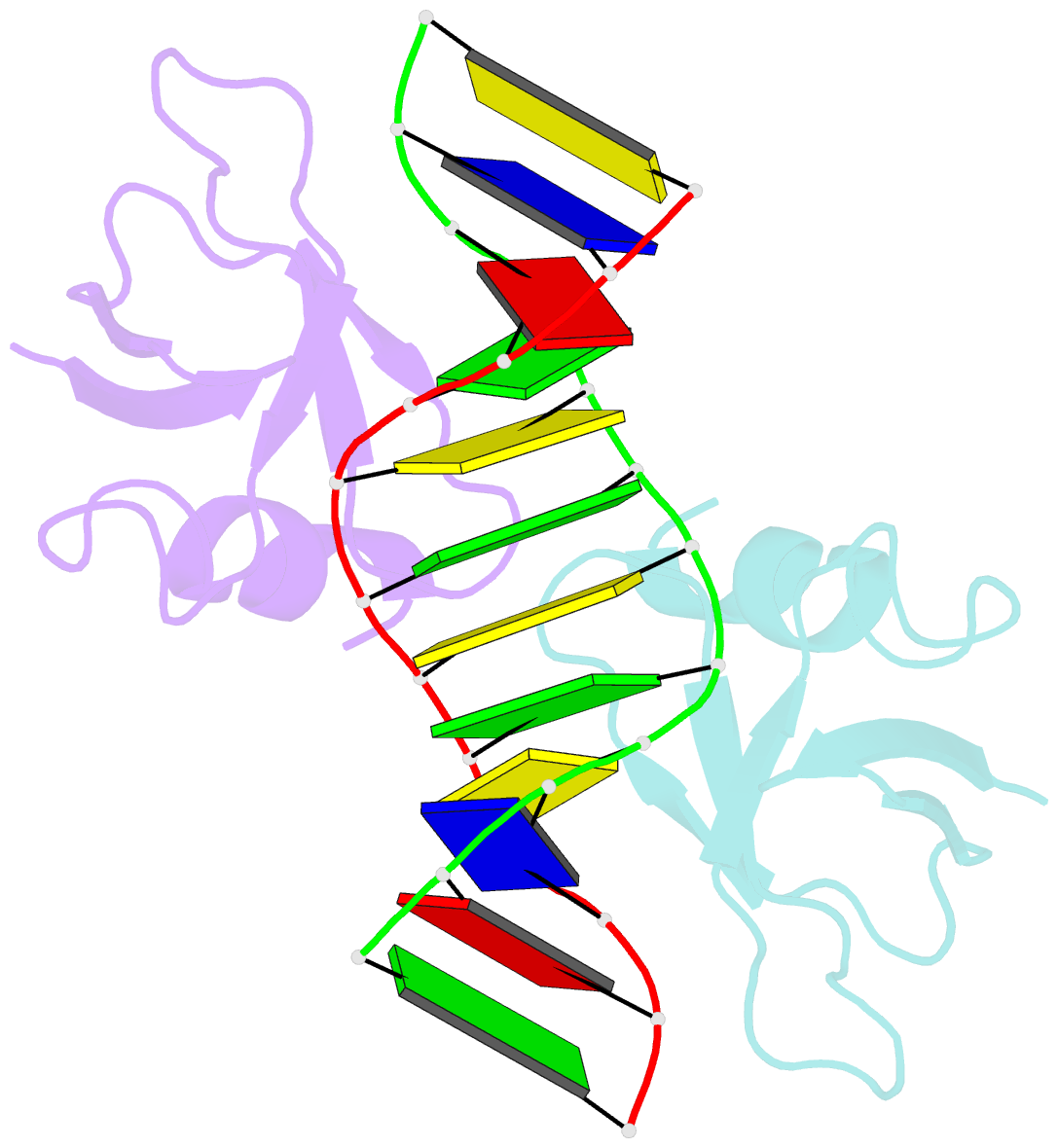
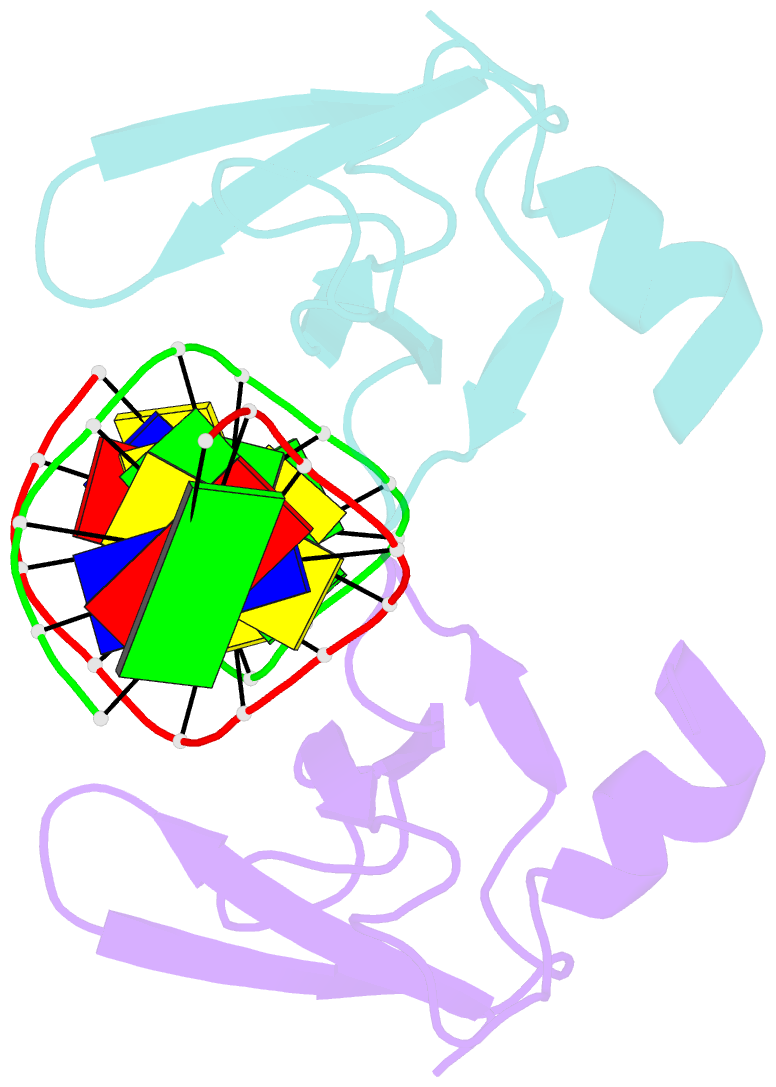
- Non-specific protein-DNA interactions in the sso7d-DNA complex, NMR, 1 structure
- Reference
- Agback P, Baumann H, Knapp S, Ladenstein R, Hard T (1998): "Architecture of Nonspecific Protein-DNA Interactions in the Sso7D-DNA Complex." Nat.Struct.Biol., 5, 579-584. doi: 10.1038/836.
- Abstract
- Many biochemical processes, including DNA packing, maintenance and control, rely on non-sequence specific protein-DNA interactions. Nonspecific DNA-binding proteins have evolved to tolerate a wide range of DNA sequences, yet bind with a respectable affinity. The nonspecific binding requirement is in contrast to that imposed on, for example, transcription factors and implies a different structural basis for the biomolecular recognition process. To address this issue, and the mechanism for archaeal DNA packing, we determined the structure of the Sso7d protein from Sulfolobus solfataricus in complex with DNA. Sso7d binds DNA by placing a triple-stranded beta-sheet across the DNA minor groove. The protein is anchored in this position by the insertion of hydrogen bond-donating side chains into the groove and additionally stabilized by electrostatic and non-polar interactions with the DNA backbone. This structure explains how strong binding can be achieved independent of DNA sequence. Sso7d binding also distorts the DNA conformation and introduces significant unwinding of the helix. This effect suggests a mechanism for DNA packing in Sulfolobus based on negative DNA supercoiling.