Summary information and primary citation
- PDB-id
- 1bdv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.8 Å)
- Summary
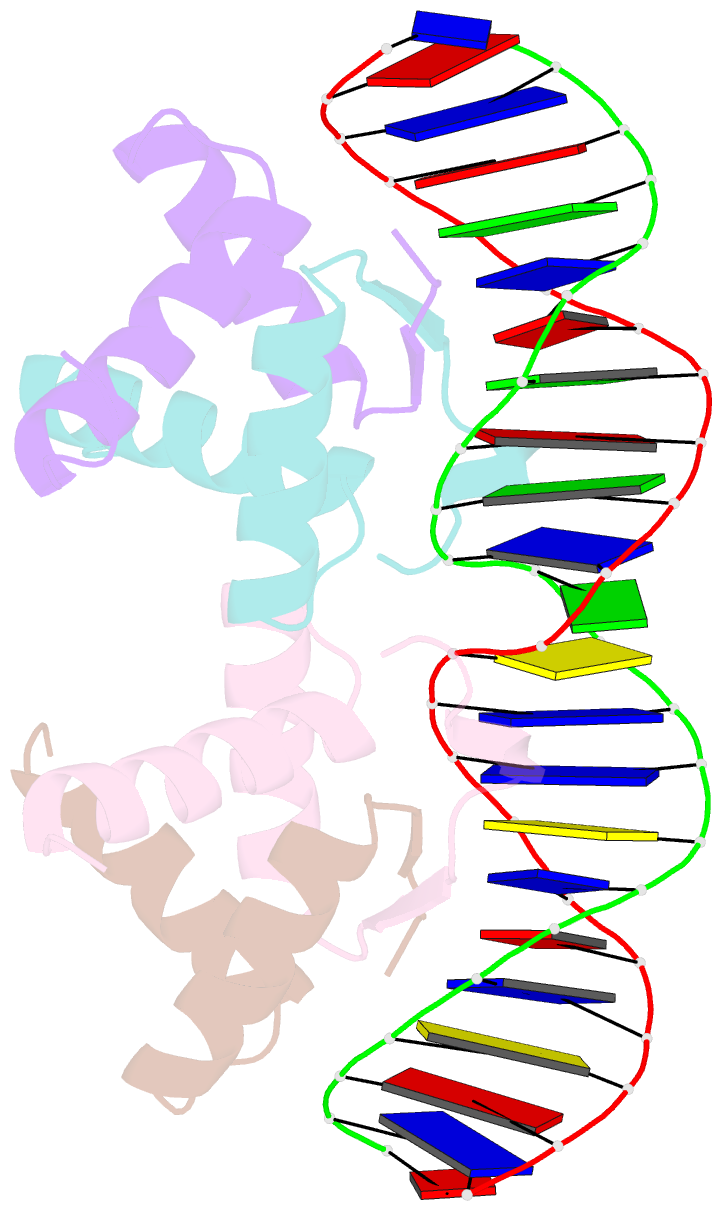
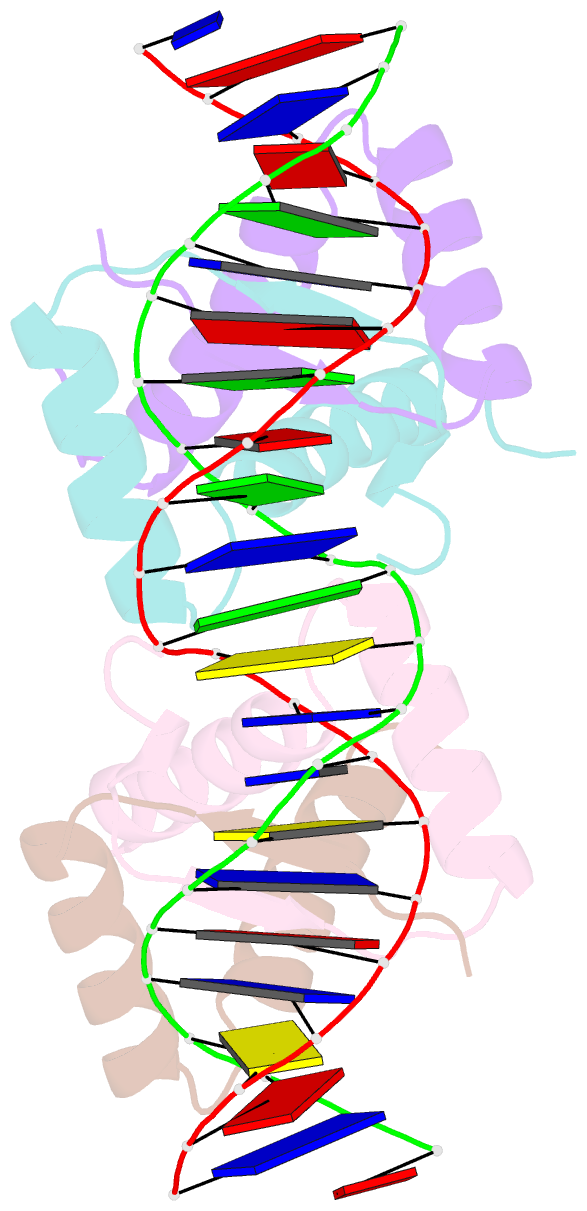
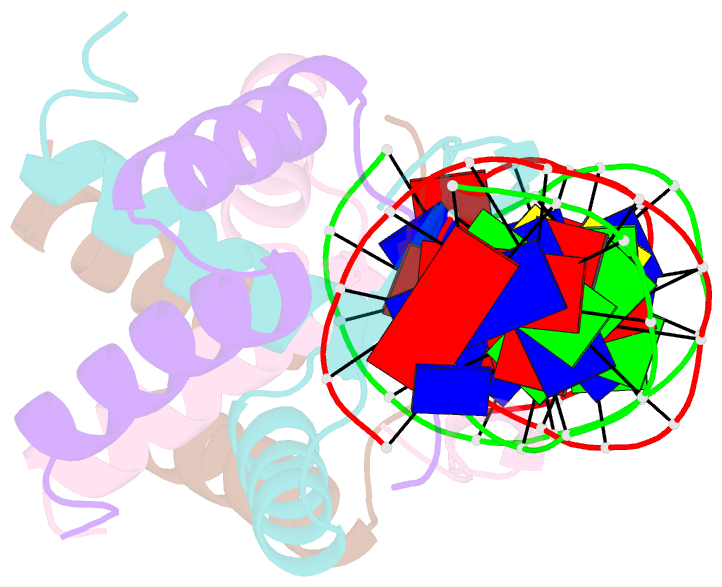
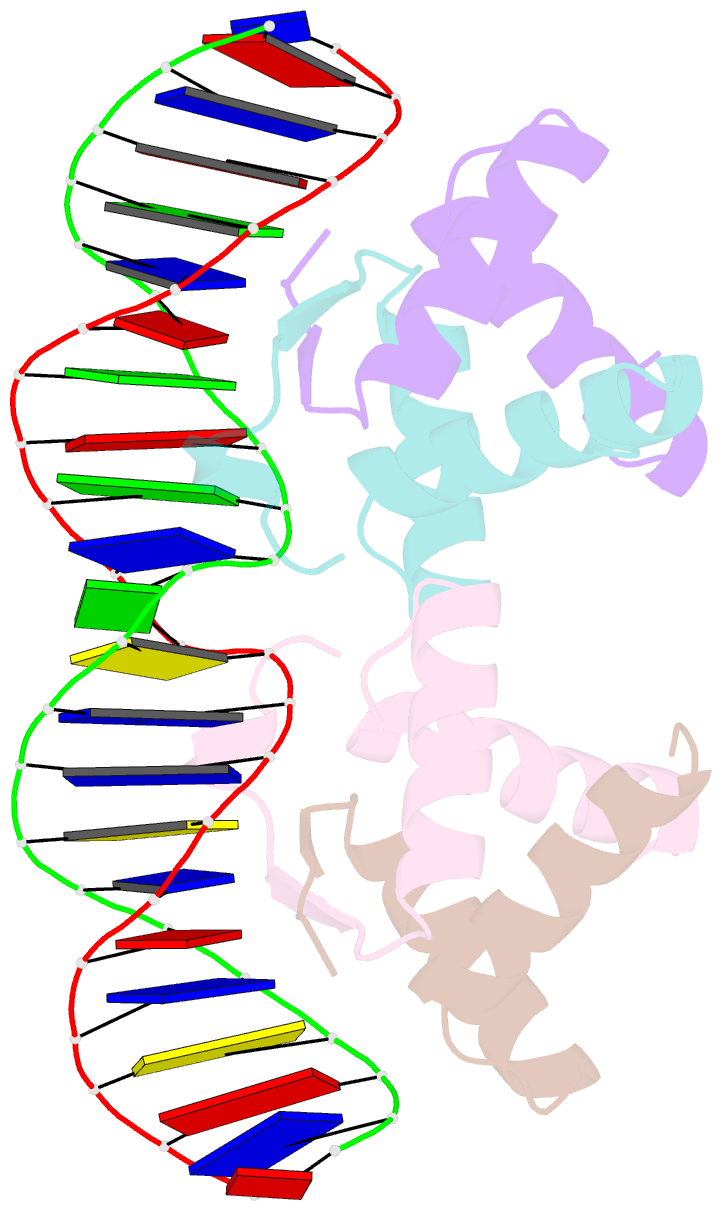
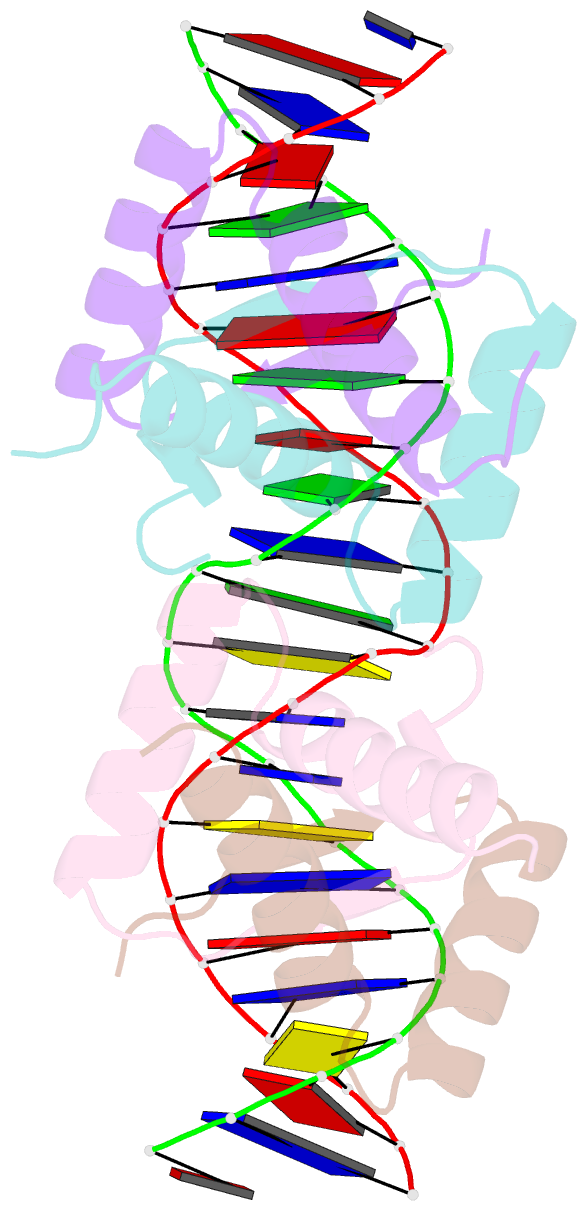
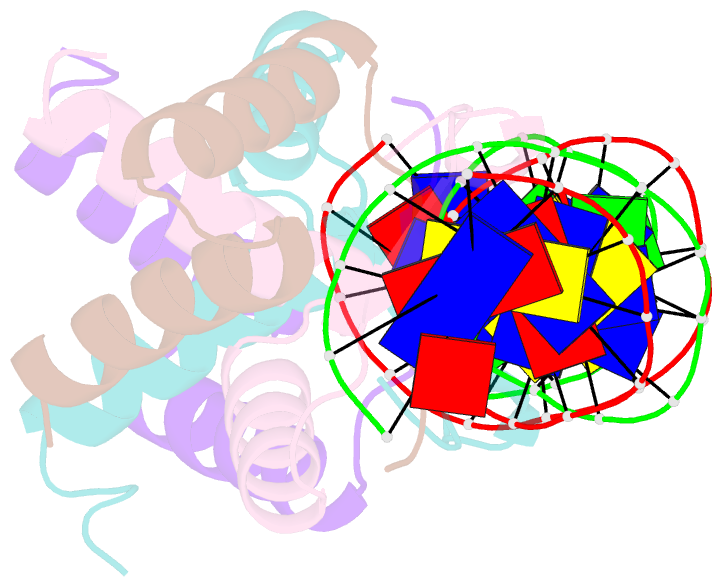
- Arc fv10 cocrystal
- Reference
- Schildbach JF, Karzai AW, Raumann BE, Sauer RT (1999): "Origins of DNA-binding specificity: role of protein contacts with the DNA backbone." Proc.Natl.Acad.Sci.USA, 96, 811-817. doi: 10.1073/pnas.96.3.811.
- Abstract
- A central question in protein-DNA recognition is the origin of the specificity that permits binding to the correct site in the presence of excess, nonspecific DNA. In the P22 Arc repressor, the Phe-10 side chain is part of the hydrophobic core of the free protein but rotates out to pack against the sugar-phosphate backbone of the DNA in the repressor-operator complex. Characterization of a library of position 10 variants reveals that Phe is the only residue that results in fully active Arc. One class of mutants folds stably but binds operator with reduced affinity; another class is unstable. FV10, one member of the first class, binds operator DNA and nonoperator DNA almost equally well. The affinity differences between FV10 and wild type indicate that each Phe-10 side chain contributes 1.5-2.0 kcal to operator binding but less than 0.5 kcal/mol to nonoperator binding, demonstrating that contacts between Phe-10 and the operator DNA backbone contribute to binding specificity. This appears to be a direct contribution as the crystal structure of the FV10 dimer is similar to wild type and the Phe-10-DNA backbone interactions are the only contacts perturbed in the cocrystal structure of the FV10-operator complex.