Summary information and primary citation
- PDB-id
- 1bmv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- virus-RNA
- Method
- X-ray (3.0 Å)
- Summary
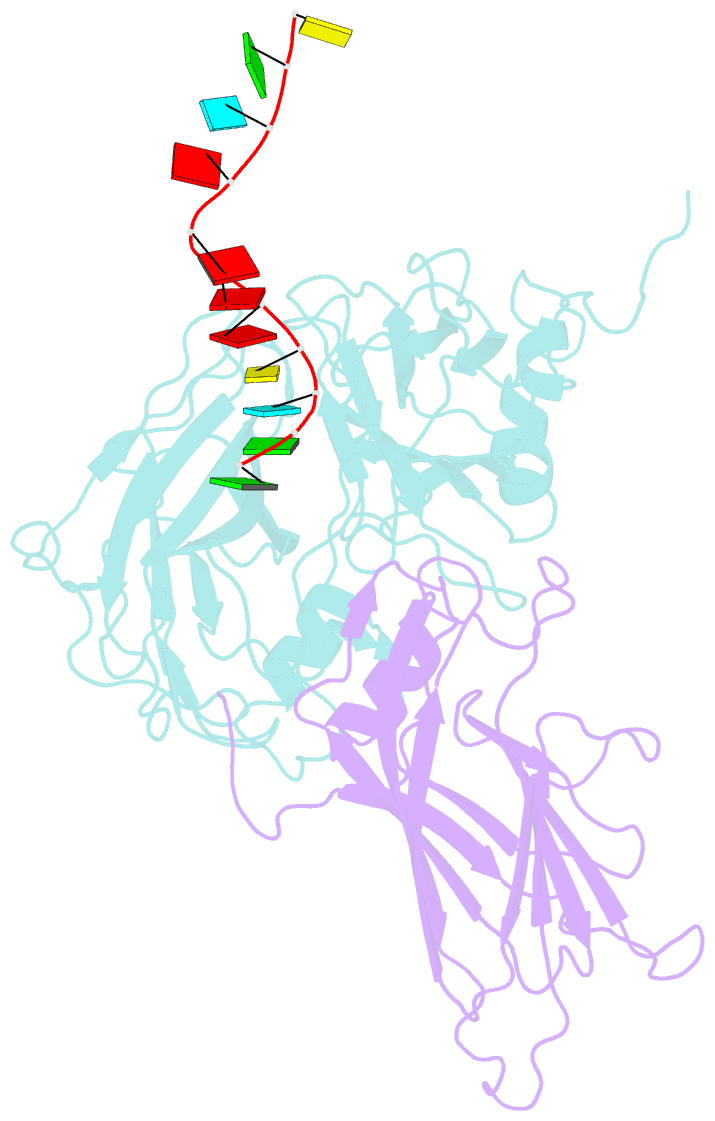
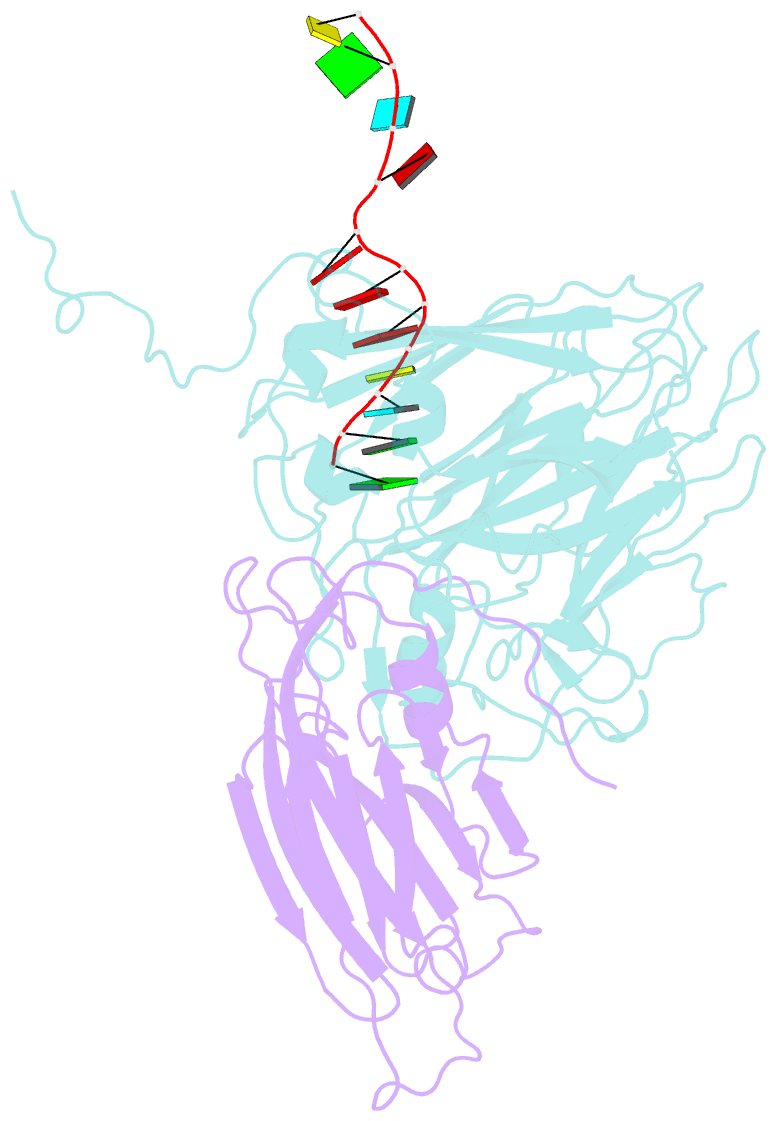
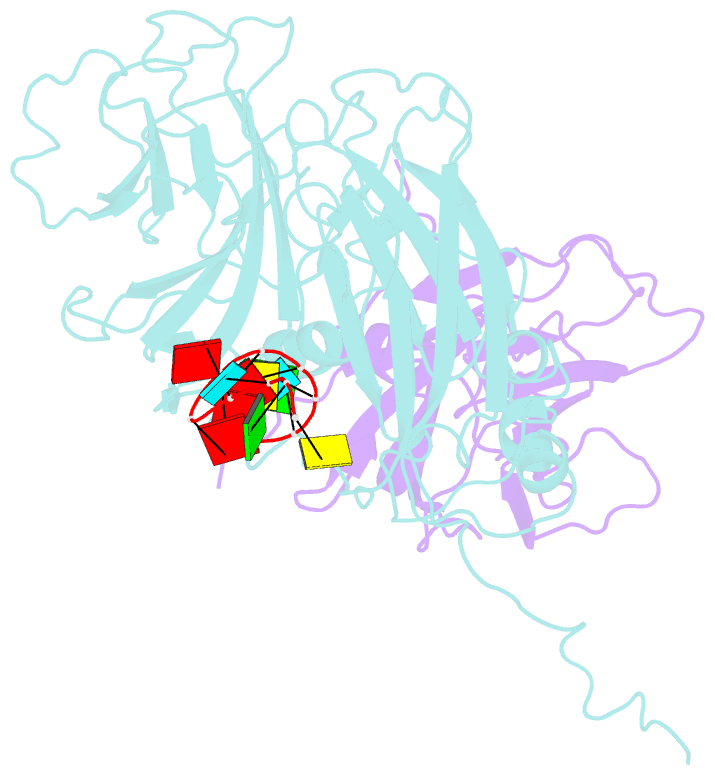
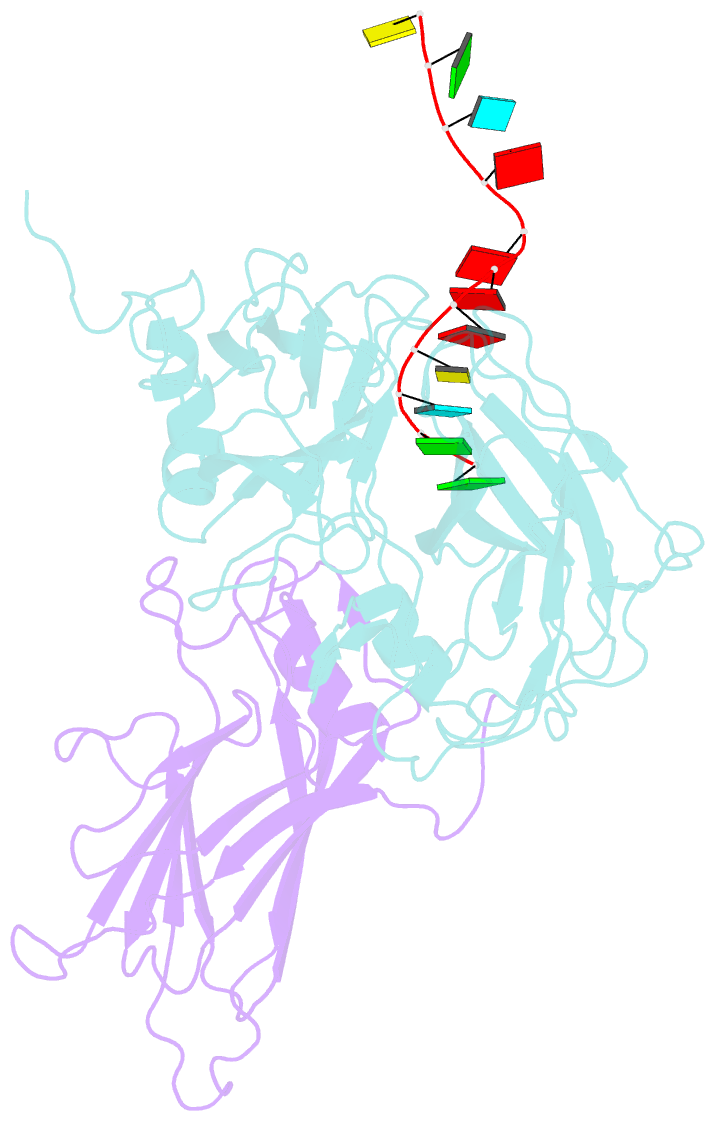
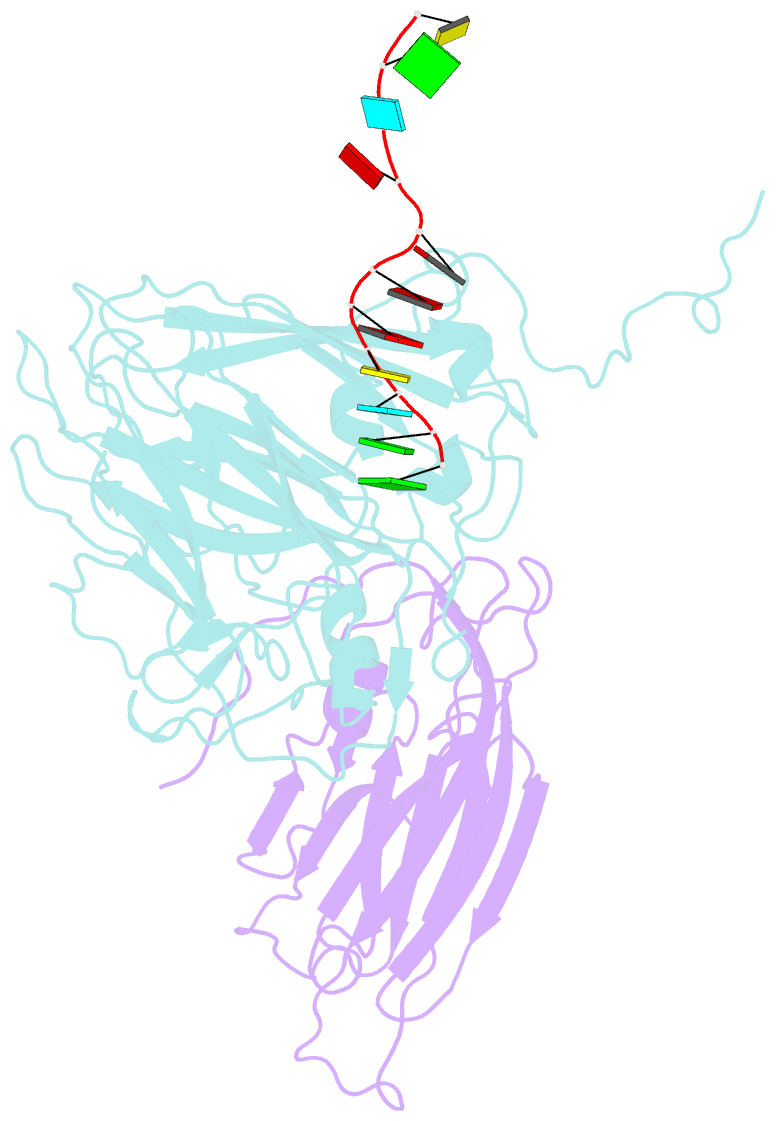
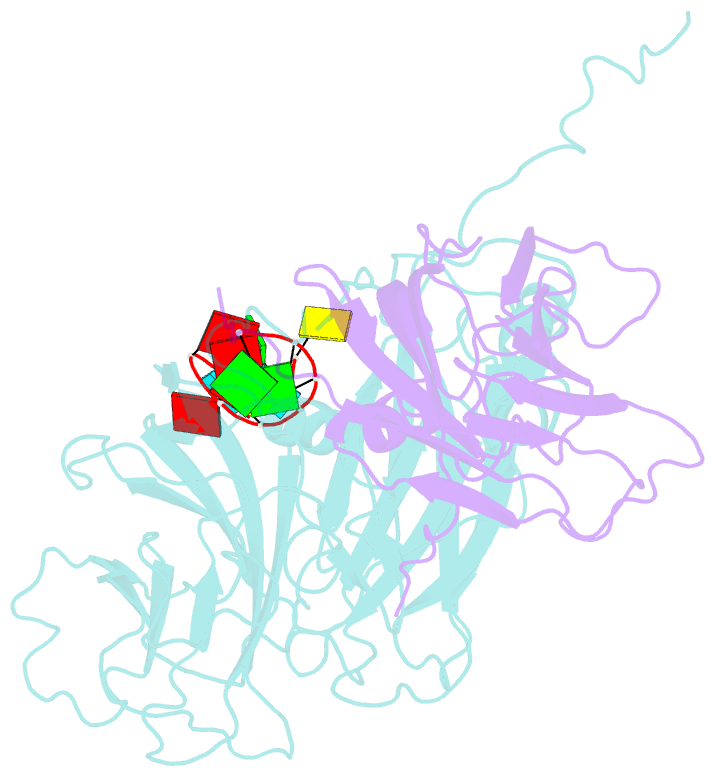
- Protein-RNA interactions in an icosahedral virus at 3.0 angstroms resolution
- Reference
- Chen ZG, Stauffacher C, Li Y, Schmidt T, Bomu W, Kamer G, Shanks M, Lomonossoff G, Johnson JE (1989): "Protein-RNA interactions in an icosahedral virus at 3.0 A resolution." Science, 245, 154-159.
- Abstract
- Nearly 20 percent of the packaged RNA in bean-pod mottle virus (BPMV) binds to the capsid interior in a symmetric fashion and is clearly visible in the electron density map. The RNA displaying icosahedral symmetry is single-stranded with well-defined polarity and stereochemical properties. Interactions with protein are dominated by nonbonding forces with few specific contacts. The tertiary and quaternary structures of the BPMV capsid proteins are similar to those observed in animal picornaviruses, supporting the close relation between plant comoviruses and animal picornaviruses established by previous biological studies.