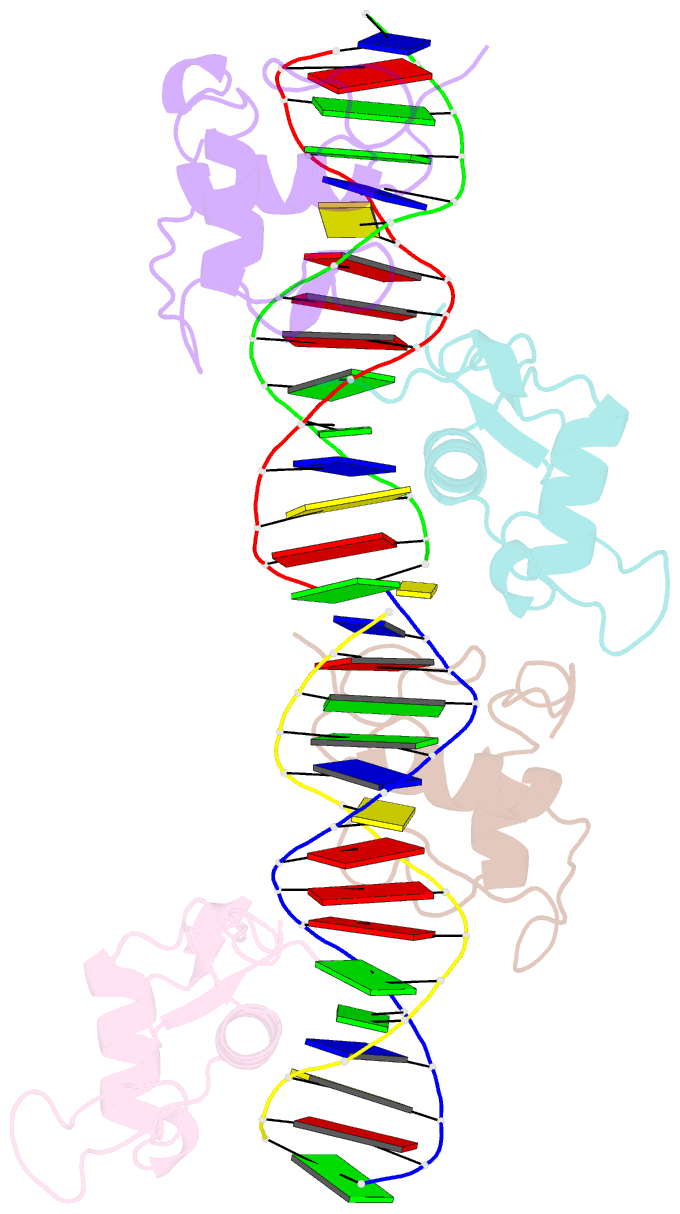
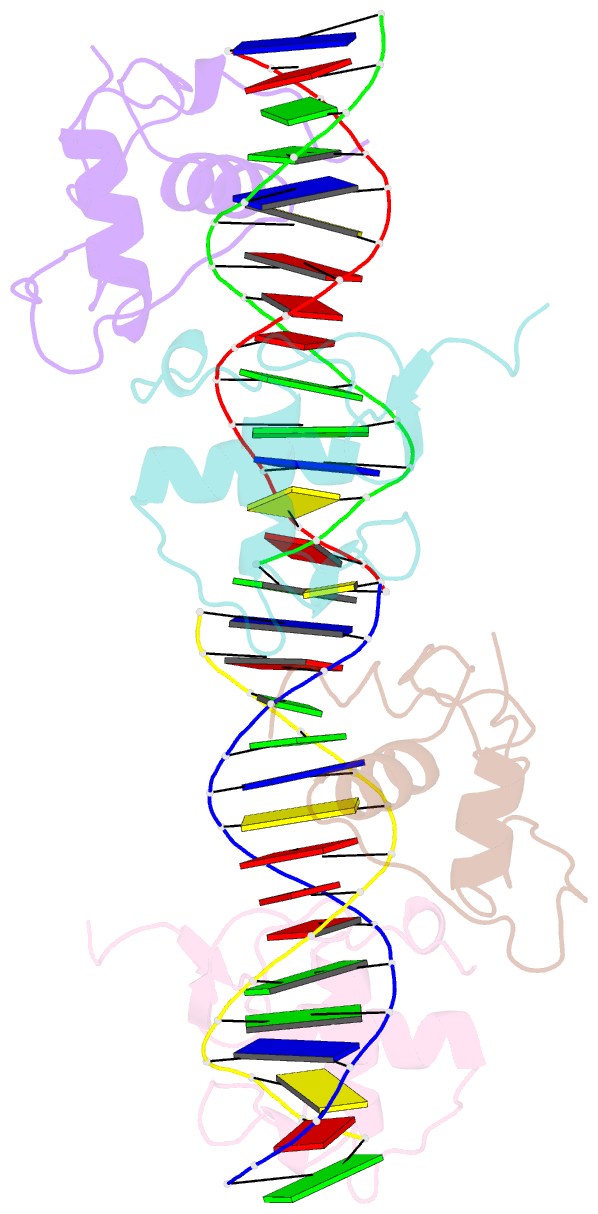
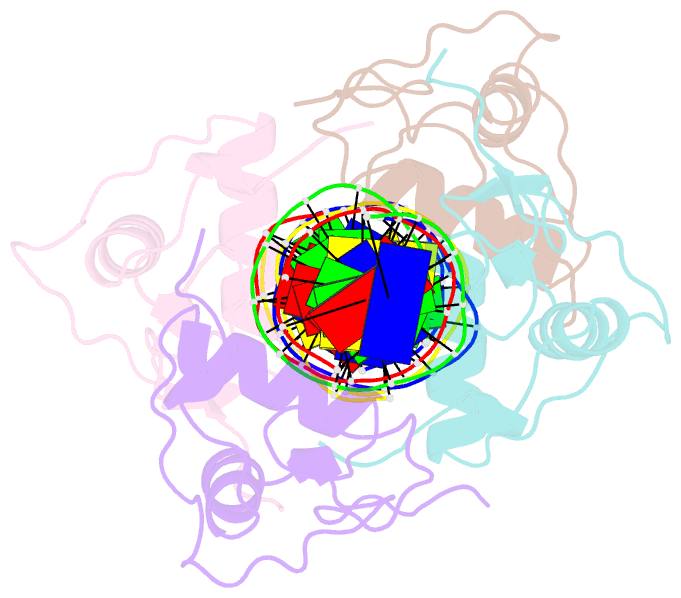
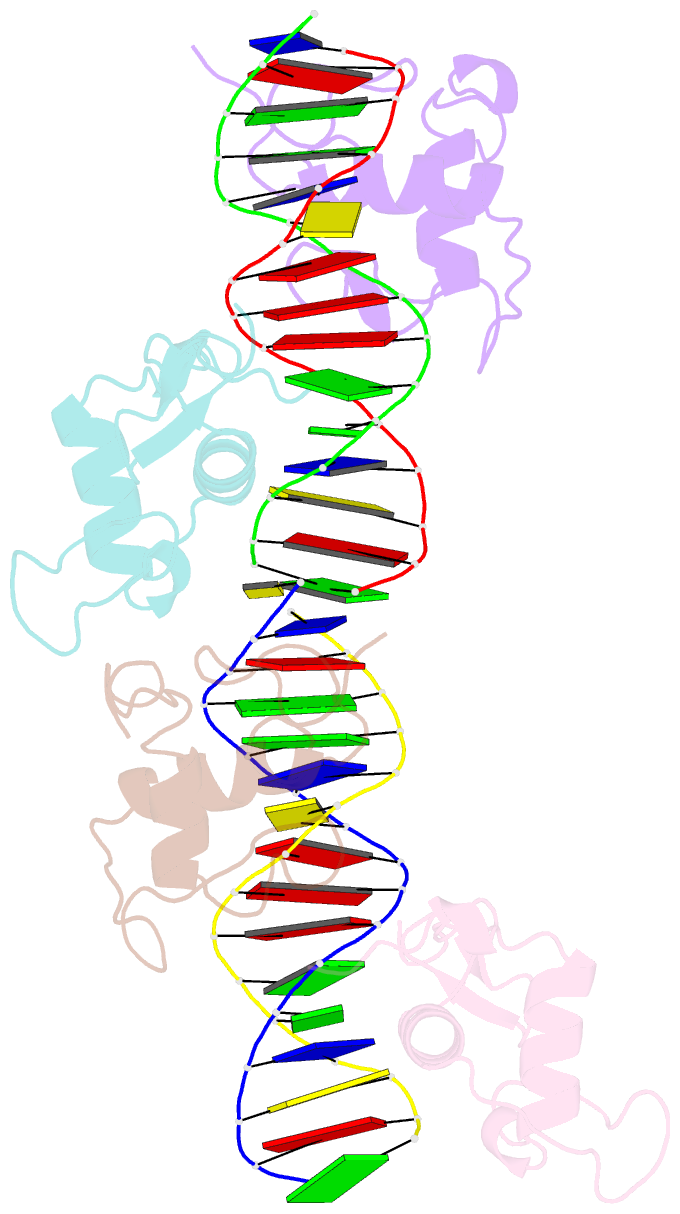
Summary information and primary citation
- PDB-id
- 1by4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-DNA
- Method
- X-ray (2.1 Å)
- Summary
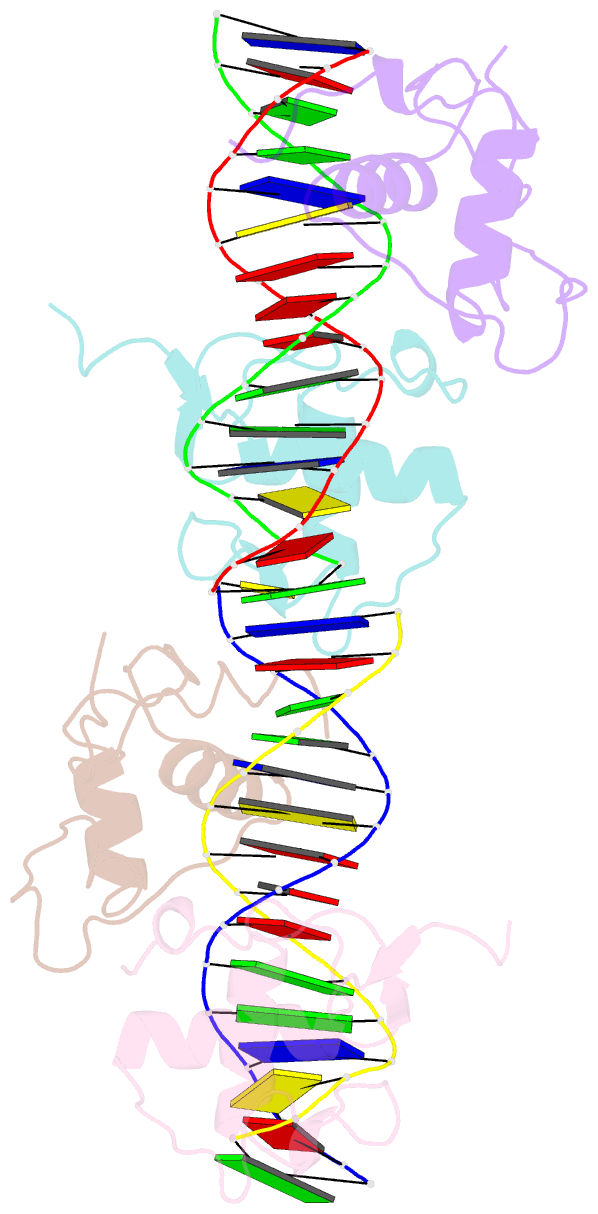
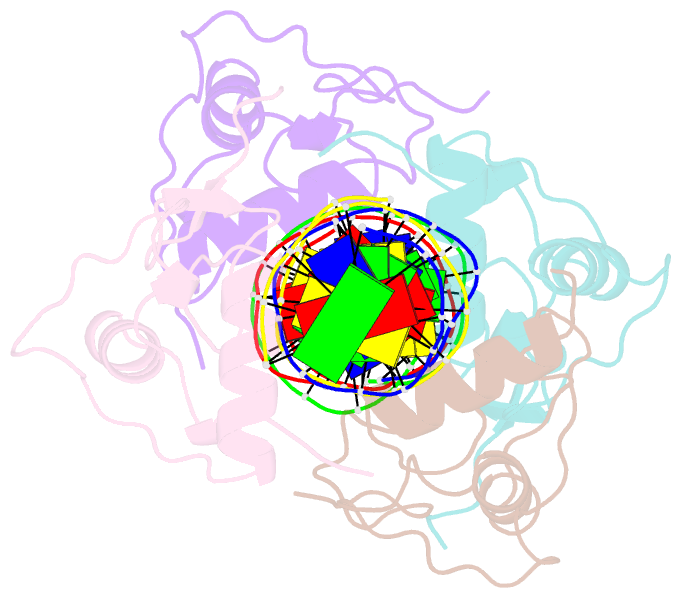
- Structure and mechanism of the homodimeric assembly of the rxr on DNA
- Reference
- Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F (2000): "Structural basis of RXR-DNA interactions." J.Mol.Biol., 296, 509-520. doi: 10.1006/jmbi.1999.3457.
- Abstract
- The 9-cis retinoic acid receptor, RXR, binds DNA effectively as a homodimer or as a heterodimer with other nuclear receptors. The DNA-binding sites for these RXR complexes are direct repeats of a consensus sequence separated by one to five base-pairs of intervening space. Here, we report the 2.1 A crystal structure of the RXR-DNA-binding domain as a homodimer in complex with its idealized direct repeat DNA target. The structure shows how a gene-regulatory site can induce conformational changes in a transcription factor that promote homo-cooperative assembly. Specifically, an alpha-helix in the T-box is disrupted to allow efficient DNA-binding and subunit dimerization. RXR displays a relaxed mode of sequence recognition, interacting with only three base-pairs in each hexameric half-site. The structure illustrates how site selection is achieved in this large eukaryotic transcription factor family through discrete protein-protein interactions and the use of tandem DNA binding sites with characteristic spacings.