Summary information and primary citation
- PDB-id
- 1c7y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- recombination-DNA
- Method
- X-ray (3.1 Å)
- Summary
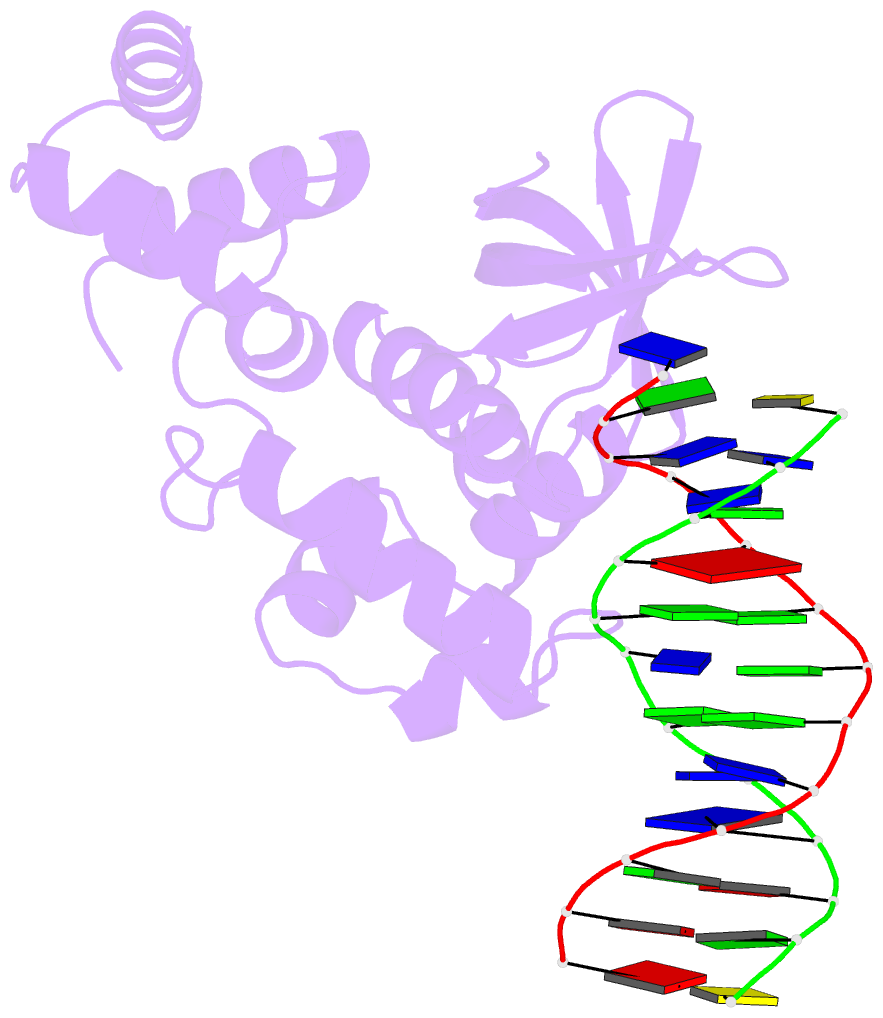
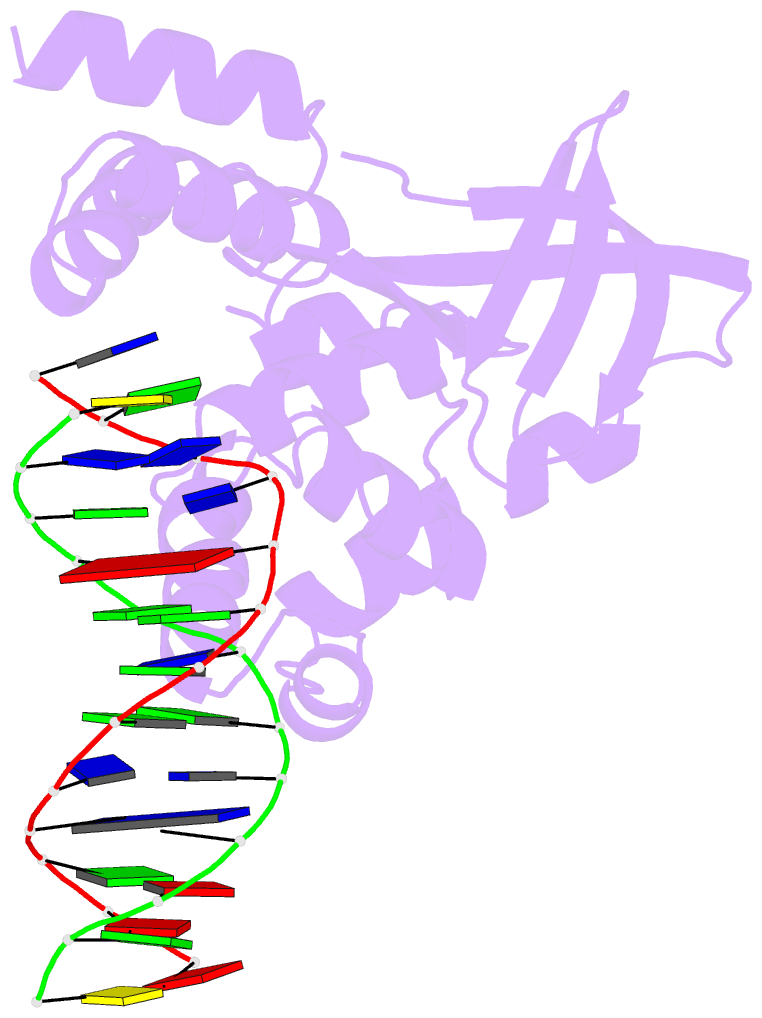
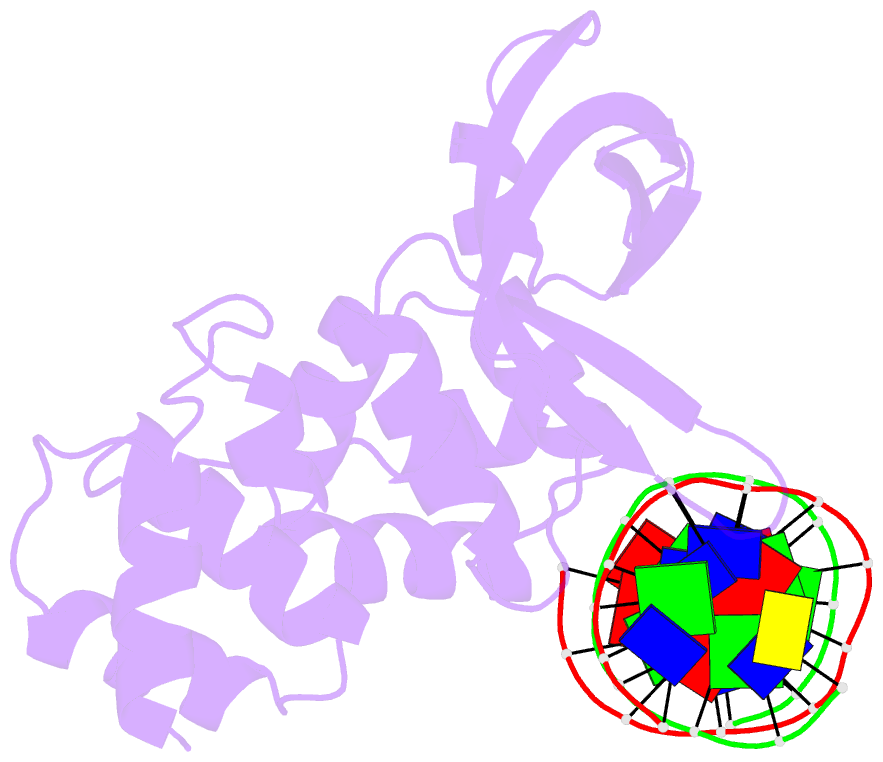
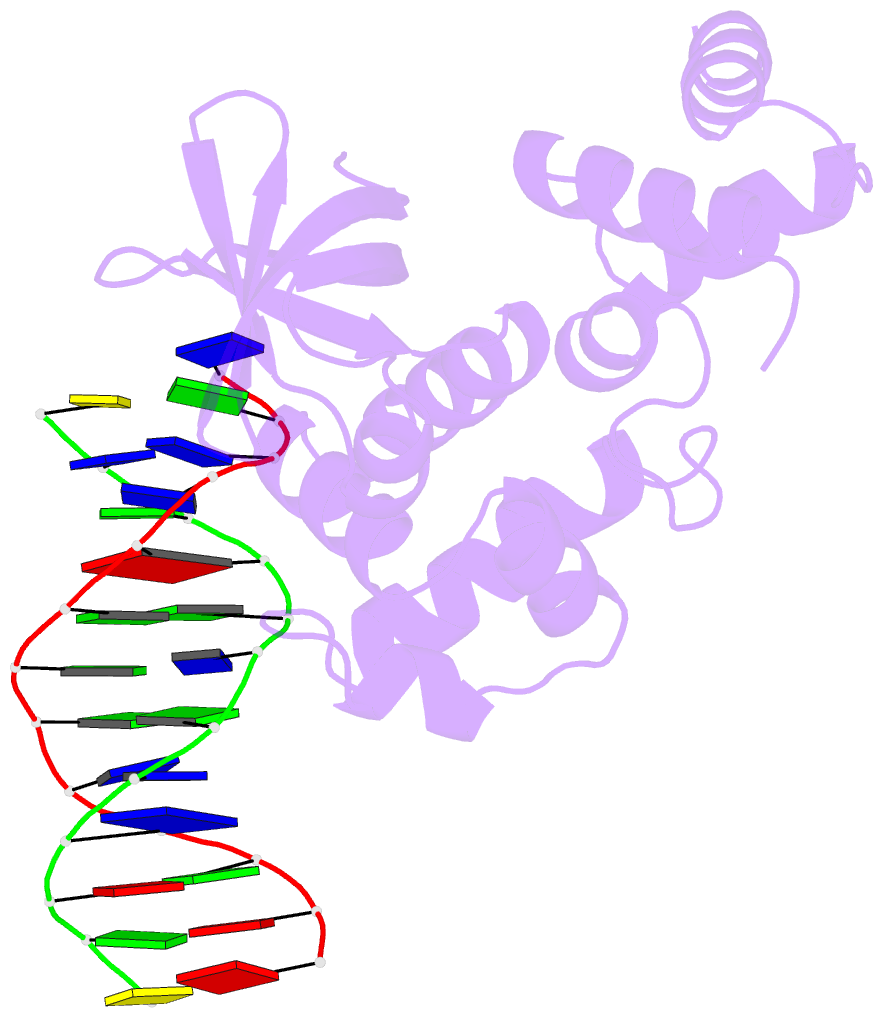
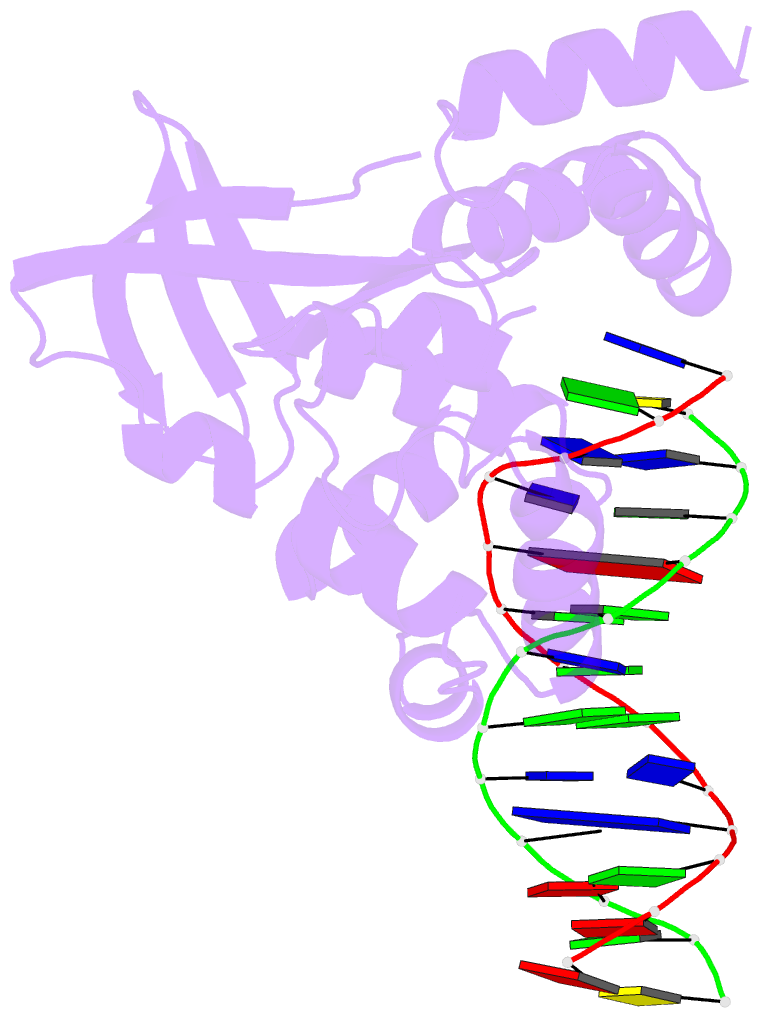
- E.coli ruva-holliday junction complex
- Reference
- Ariyoshi M, Nishino T, Iwasaki H, Shinagawa H, Morikawa K (2000): "Crystal structure of the holliday junction DNA in complex with a single RuvA tetramer." Proc.Natl.Acad.Sci.USA, 97, 8257-8262. doi: 10.1073/pnas.140212997.
- Abstract
- In the major pathway of homologous DNA recombination in prokaryotic cells, the Holliday junction intermediate is processed through its association with RuvA, RuvB, and RuvC proteins. Specific binding of the RuvA tetramer to the Holliday junction is required for the RuvB motor protein to be loaded onto the junction DNA, and the RuvAB complex drives the ATP-dependent branch migration. We solved the crystal structure of the Holliday junction bound to a single Escherichia coli RuvA tetramer at 3.1-A resolution. In this complex, one side of DNA is accessible for cleavage by RuvC resolvase at the junction center. The refined junction DNA structure revealed an open concave architecture with a four-fold symmetry. Each arm, with B-form DNA, in the Holliday junction is predominantly recognized in the minor groove through hydrogen bonds with two repeated helix-hairpin-helix motifs of each RuvA subunit. The local conformation near the crossover point, where two base pairs are disrupted, suggests a possible scheme for successive base pair rearrangements, which may account for smooth Holliday junction movement without segmental unwinding.