Summary information and primary citation
- PDB-id
- 1c9s; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.9 Å)
- Summary
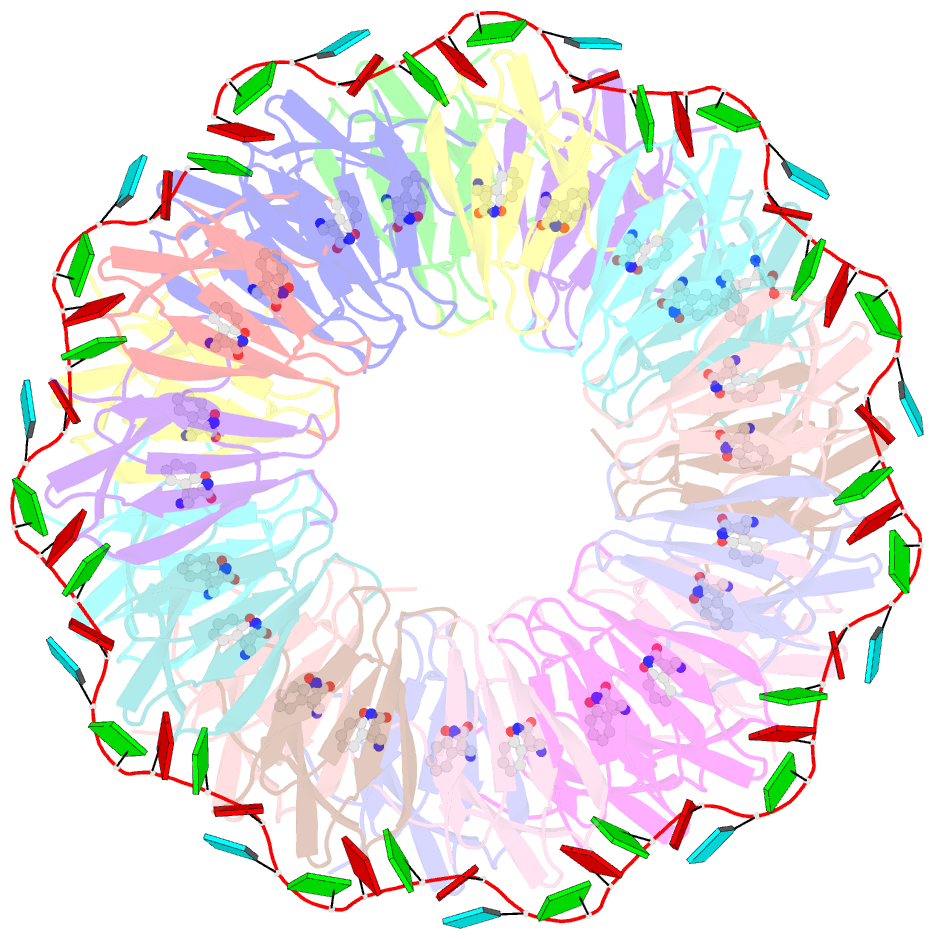
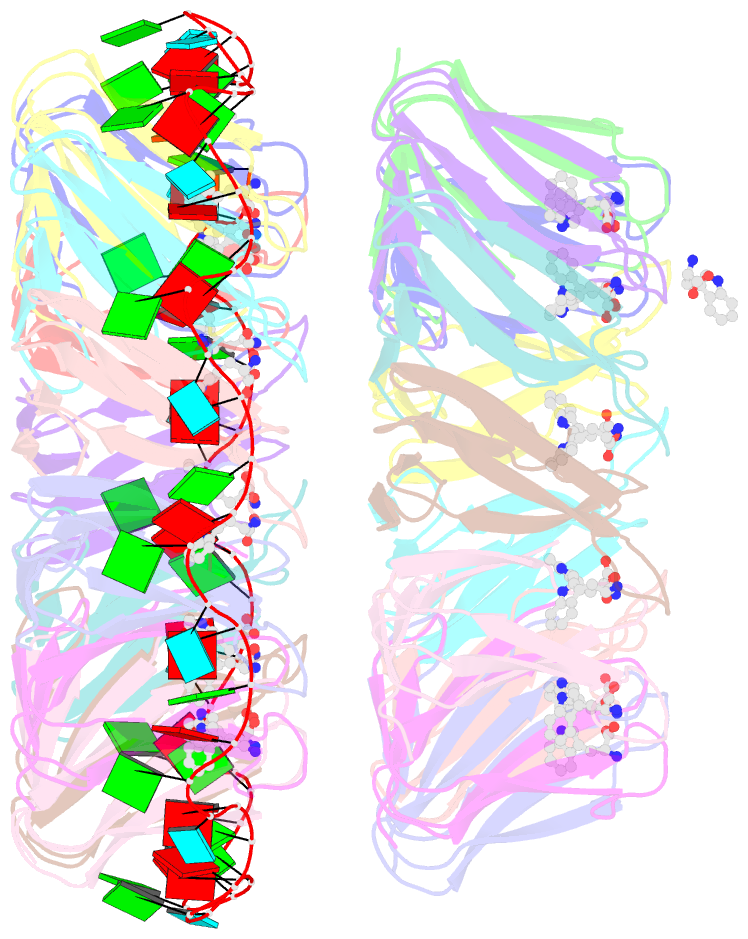
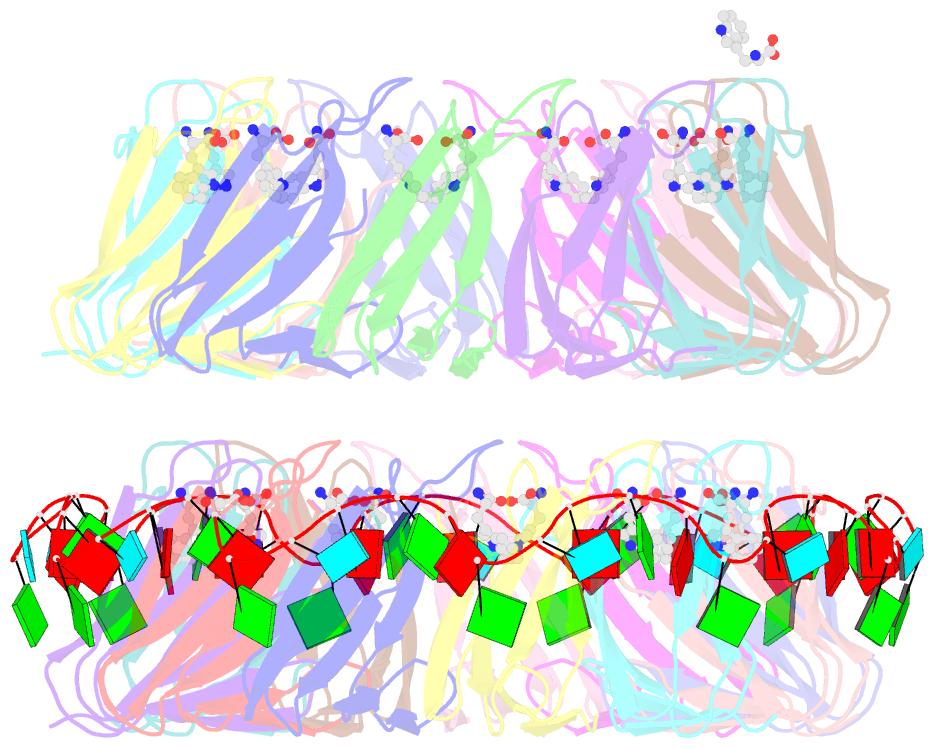
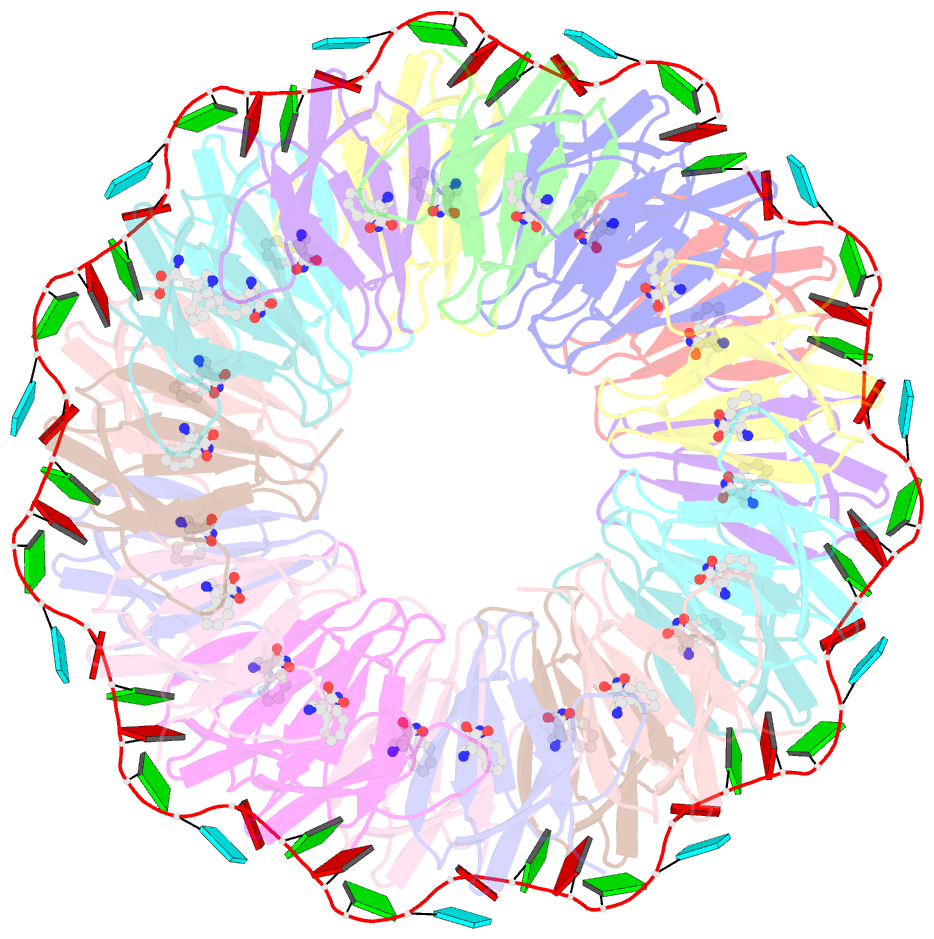
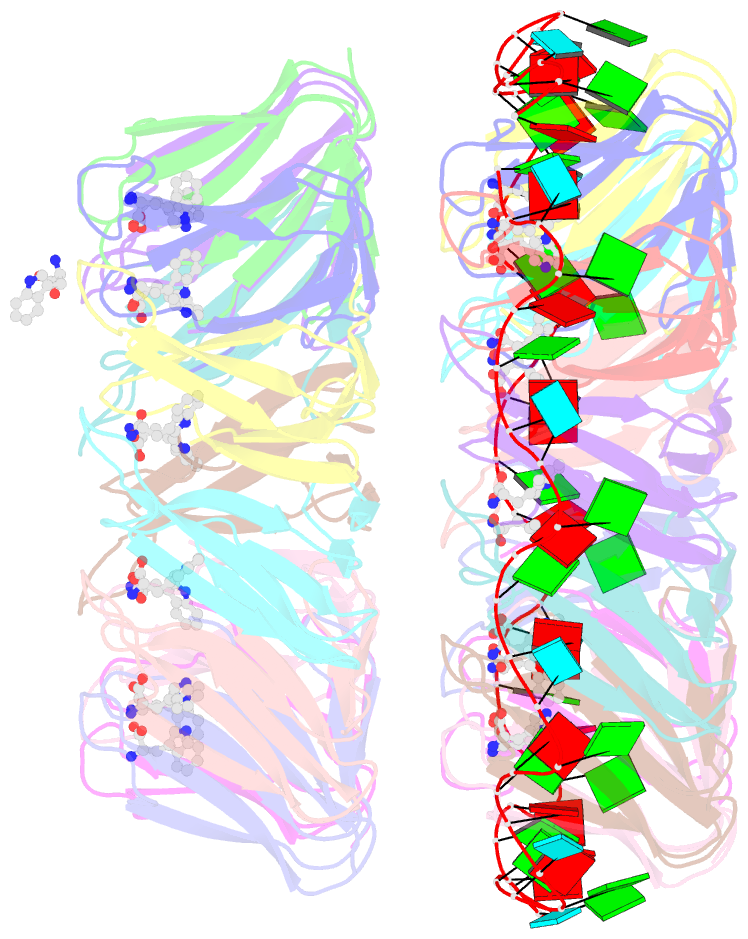
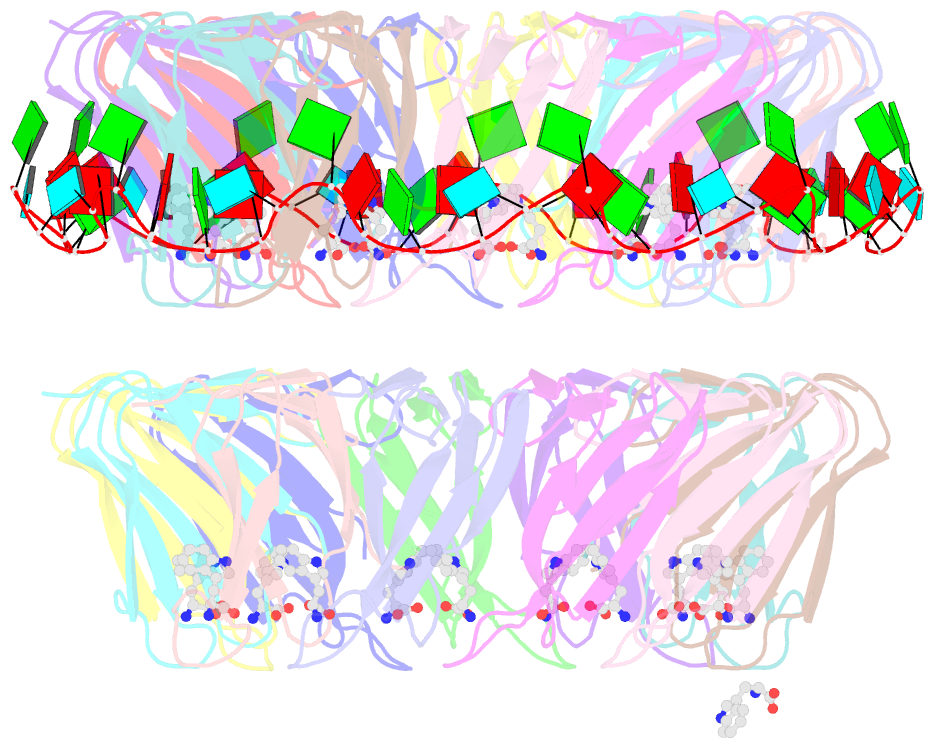
- Crystal structure of a complex of trp RNA-binding attenuation protein with a 53-base single stranded RNA containing eleven gag triplets separated by au dinucleotides
- Reference
- Antson AA, Dodson EJ, Dodson G, Greaves RB, Chen X, Gollnick P (1999): "Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA." Nature, 401, 235-242. doi: 10.1038/45730.
- Abstract
- The trp RNA-binding attenuation protein (TRAP) regulates expression of the tryptophan biosynthetic genes of several bacilli by binding single-stranded RNA. The binding sequence is composed of eleven triplet repeats, predominantly GAG, separated by two or three non-conserved nucleotides. Here we present the crystal structure of a complex of TRAP and a 53-base single-stranded RNA containing eleven GAG triplets, revealing that each triplet is accommodated in a binding pocket formed by beta-strands. In the complex, the RNA has an extended structure without any base-pairing and binds to the protein mostly by specific protein-base interactions. Eleven binding pockets on the circular TRAP 11-mer form a belt with a diameter of about 80 A. This simple but elegant mechanism of arresting the RNA segment by encircling it around a protein disk is applicable to both transcription, when TRAP binds the nascent RNA, and to translation, when TRAP binds the same sequence within a non-coding leader region of the messenger RNA.