Summary information and primary citation
- PDB-id
- 1clq; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.7 Å)
- Summary
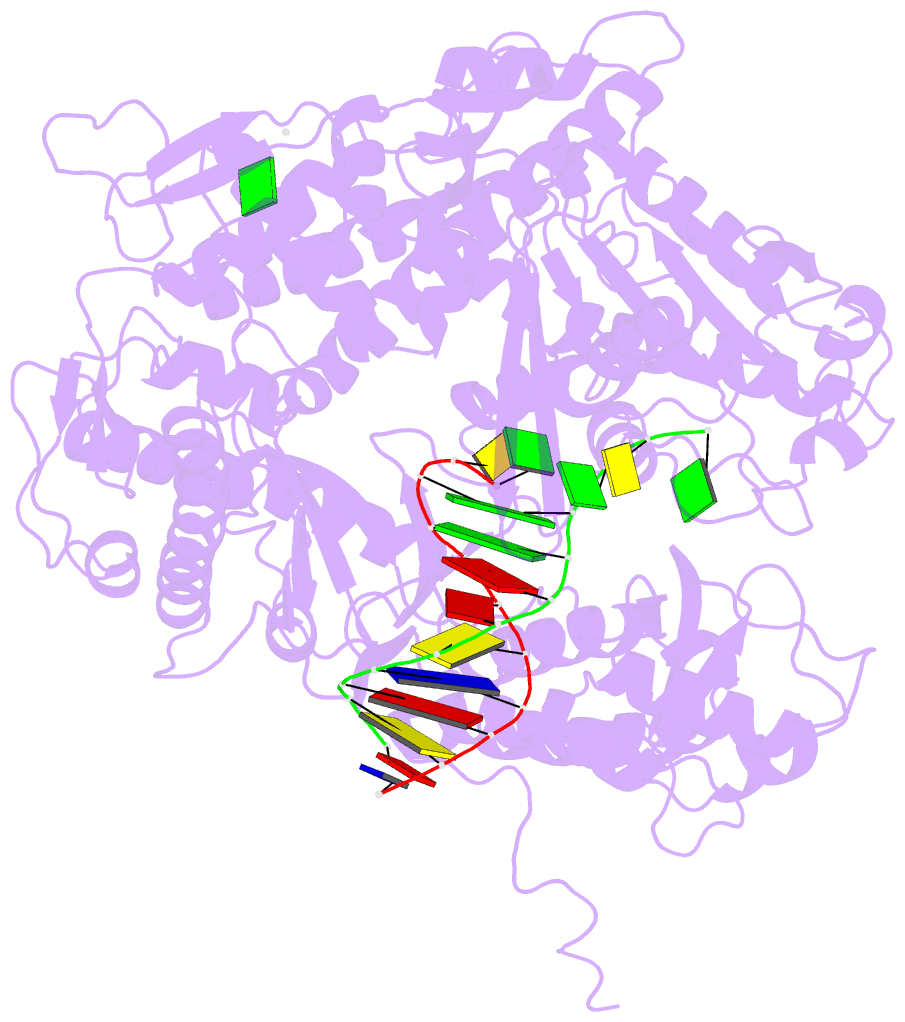
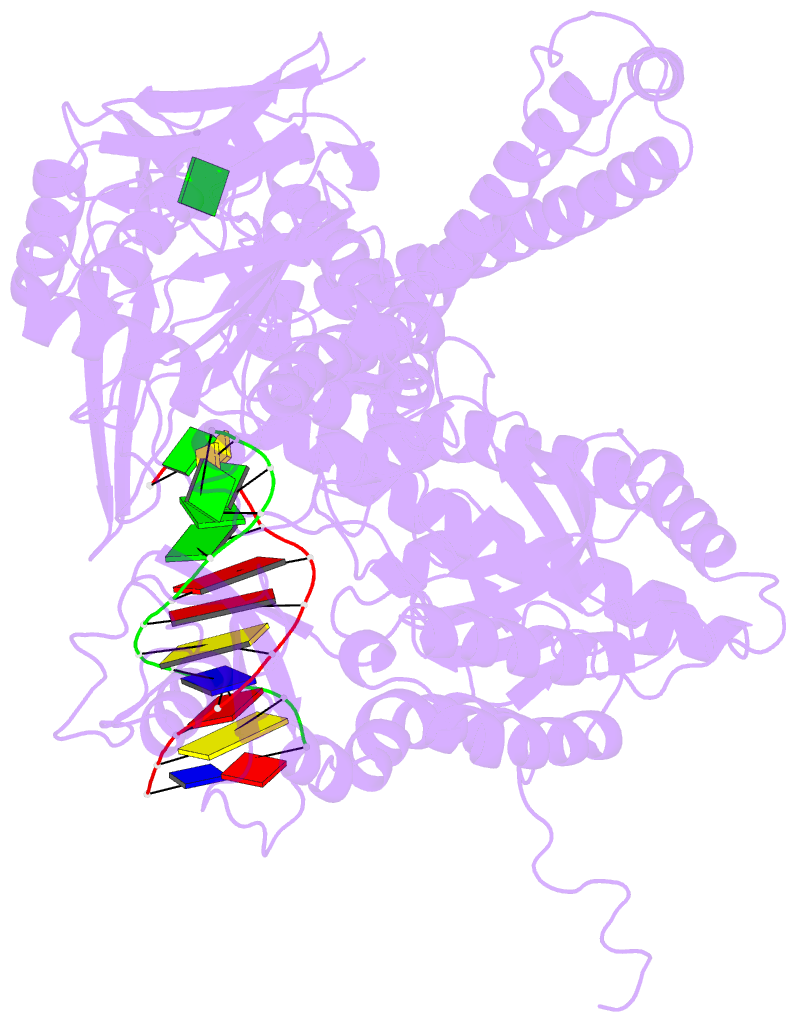
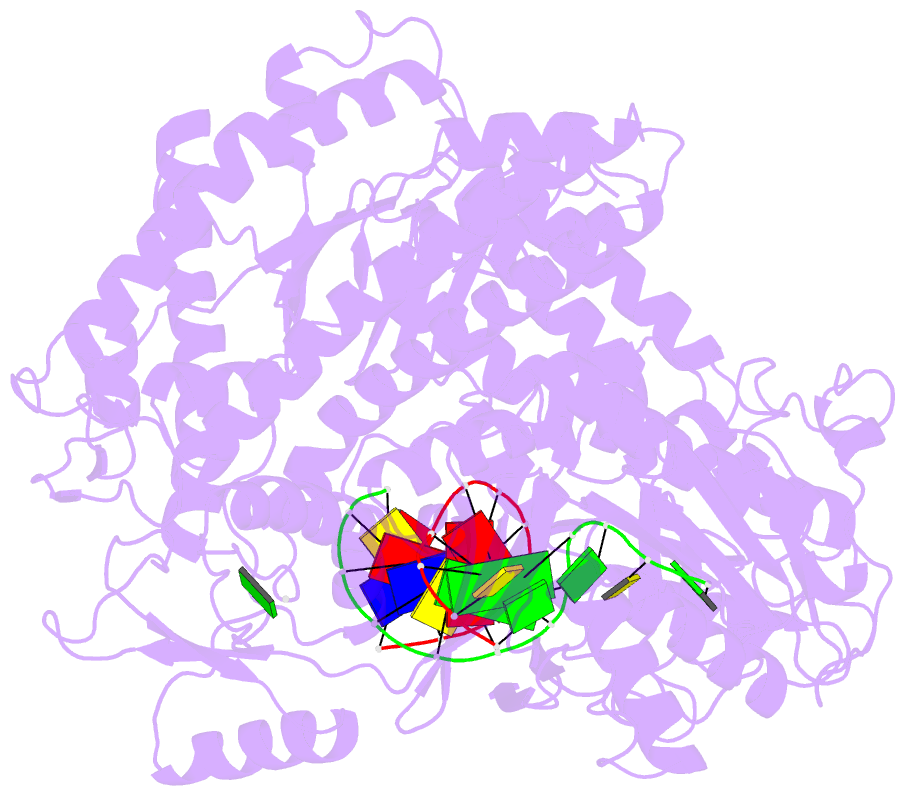
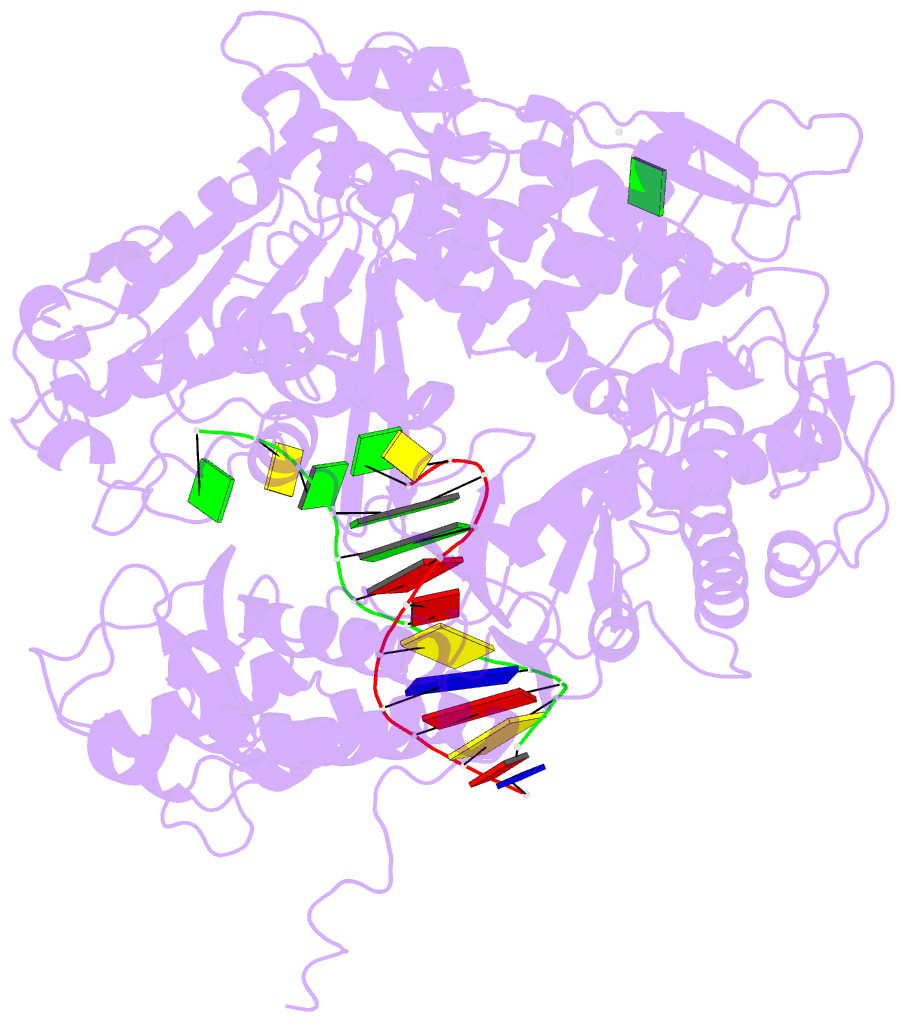
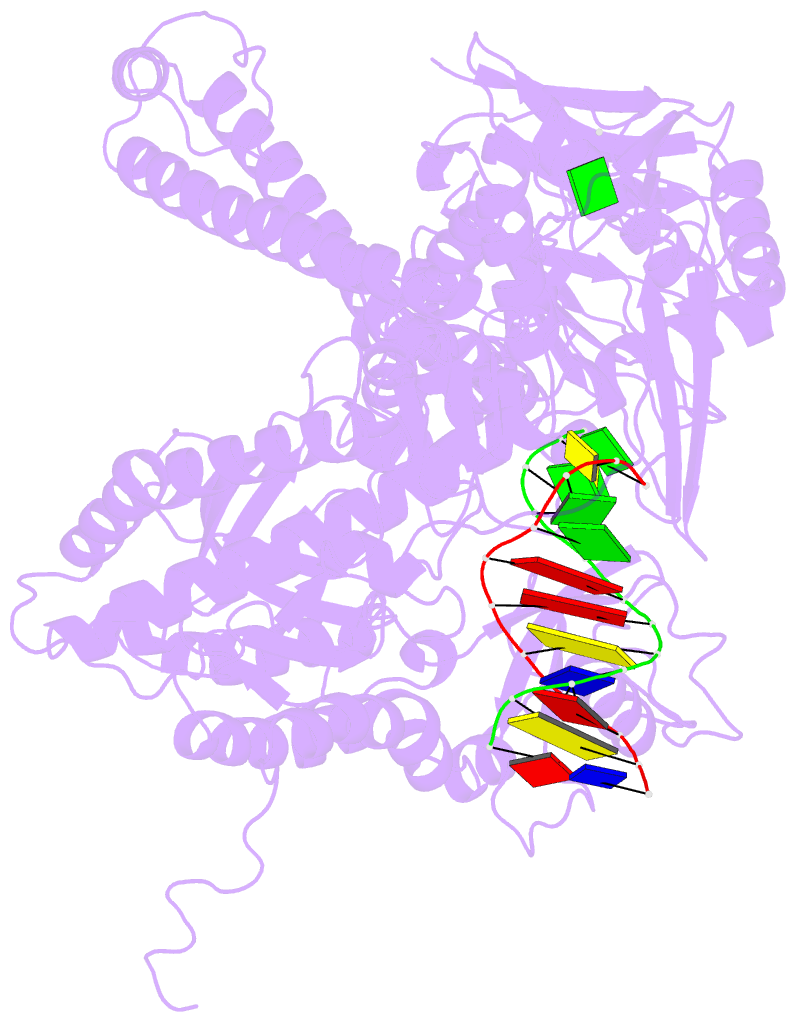
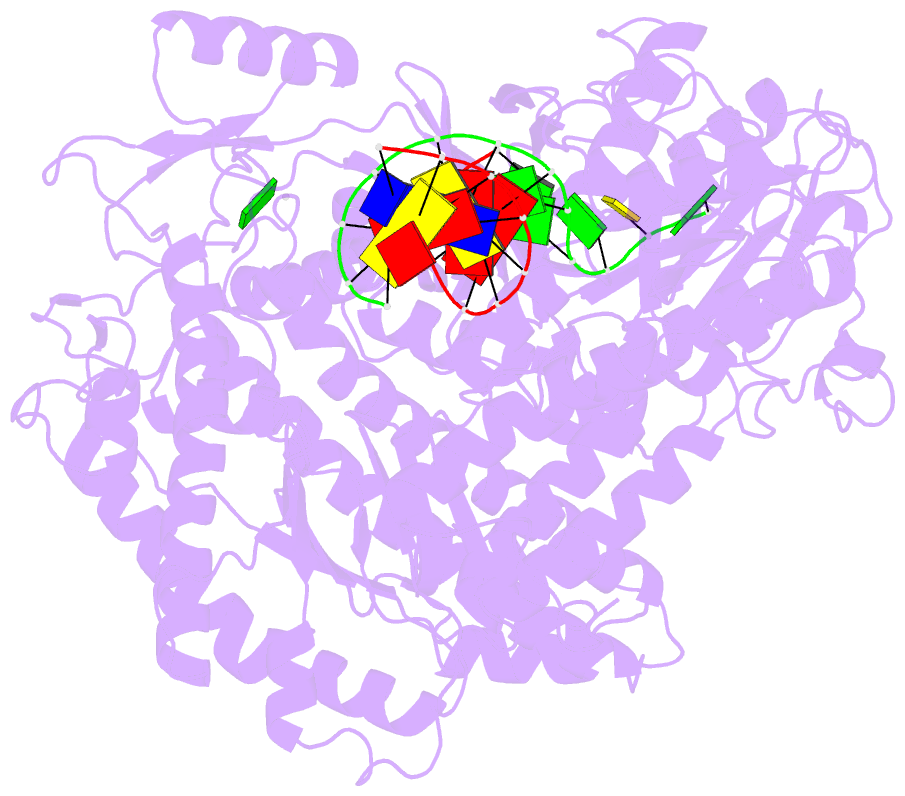
- Crystal structure of a replication fork DNA polymerase editing complex at 2.7 Å resolution
- Reference
- Shamoo Y, Steitz TA (1999): "Building a replisome from interacting pieces: sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex." Cell(Cambridge,Mass.), 99, 155-166. doi: 10.1016/S0092-8674(00)81647-5.
- Abstract
- We have solved the crystal structures of the bacteriophage RB69 sliding clamp, its complex with a peptide essential for DNA polymerase interactions, and the DNA polymerase complexed with primer-template DNA. The editing complex structure shows a partially melted duplex DNA exiting from the exonuclease domain at an unexpected angle and significant changes in the protein structure. The clamp complex shows the C-terminal 11 residues of polymerase bound in a hydrophobic pocket, and it allows docking of the editing and clamp structures together. The peptide binds to the sliding clamp at a position identical to that of a replication inhibitor peptide bound to PCNA, suggesting that the replication inhibitor protein p21CIP1 functions by competing with eukaryotic polymerases for the same binding pocket on the clamp.