Summary information and primary citation
- PDB-id
- 1crx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication-DNA
- Method
- X-ray (2.4 Å)
- Summary
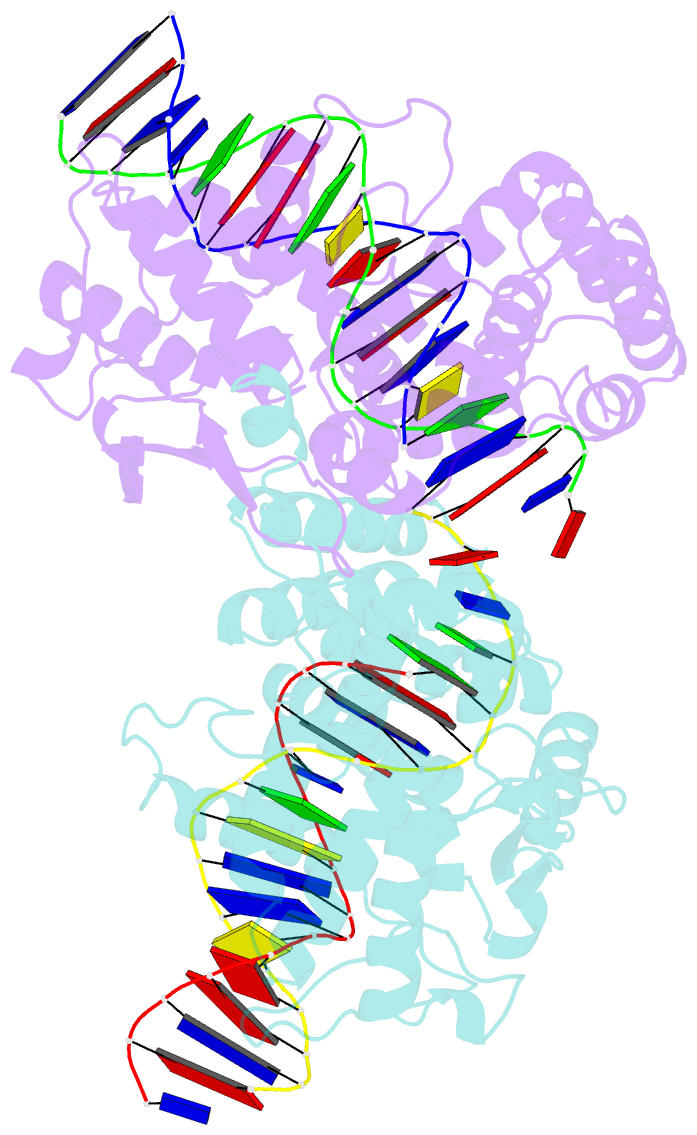
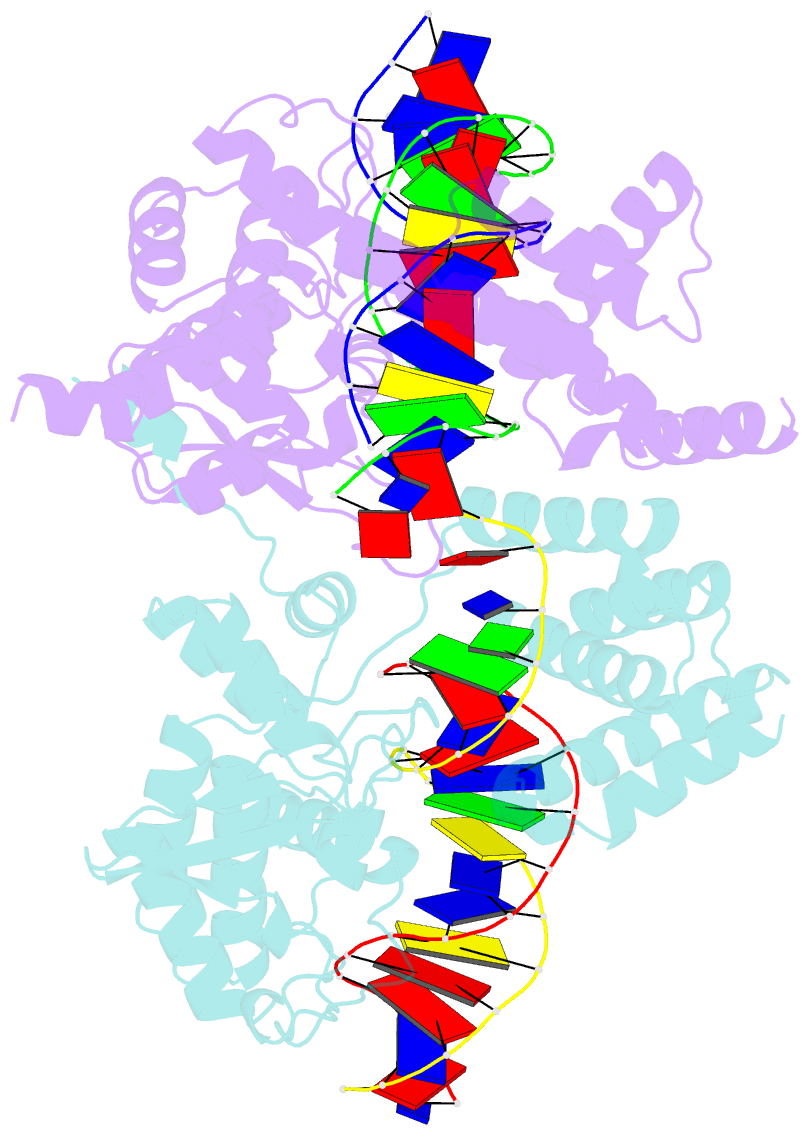
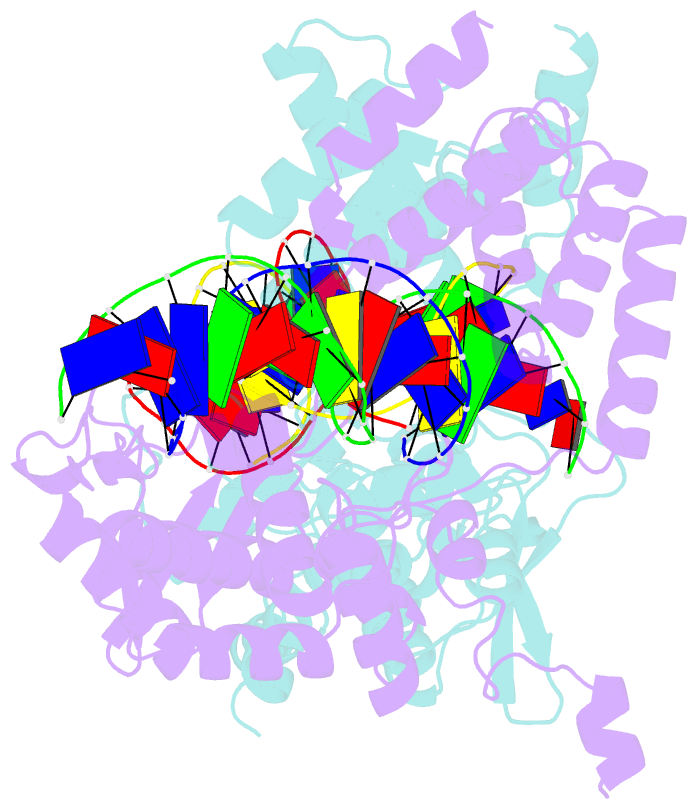
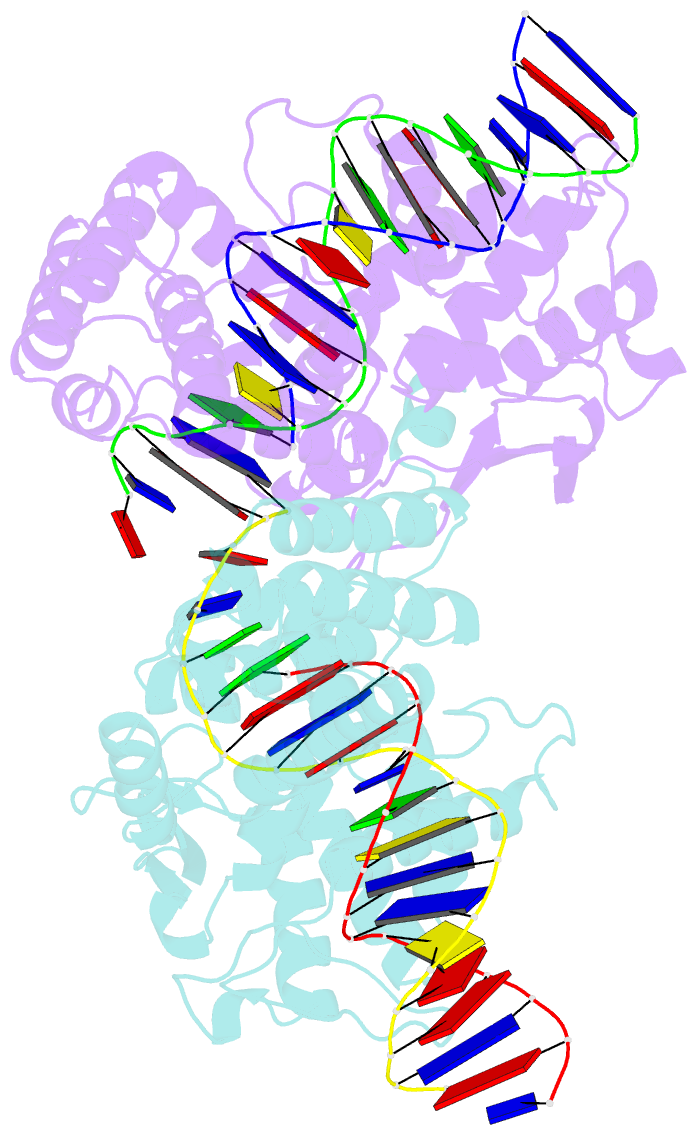
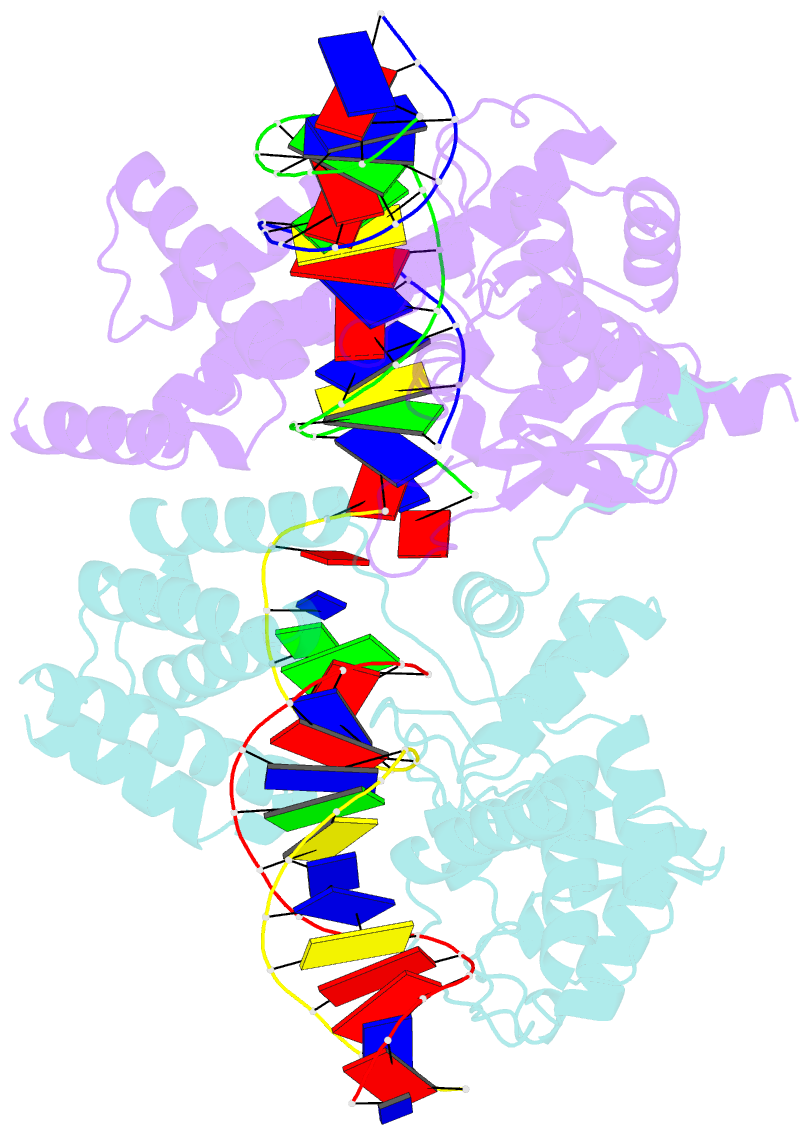
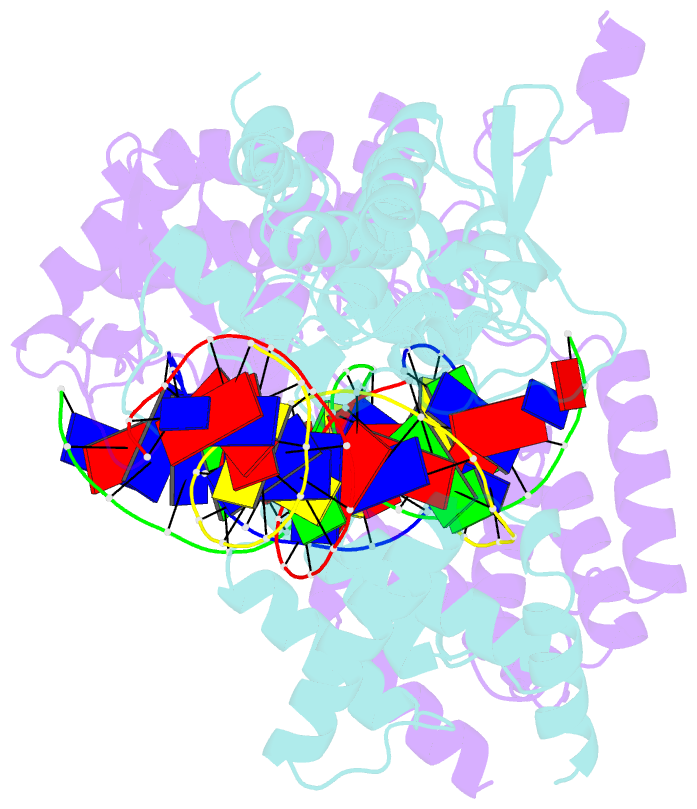
- Cre recombinase-DNA complex reaction intermediate i
- Reference
- Guo F, Gopaul DN, van Duyne GD (1997): "Structure of Cre recombinase complexed with DNA in a site-specific recombination synapse." Nature, 389, 40-46. doi: 10.1038/37925.
- Abstract
- During site-specific DNA recombination, which brings about genetic rearrangement in processes such as viral integration and excision and chromosomal segregation, recombinase enzymes recognize specific DNA sequences and catalyse the reciprocal exchange of DNA strands between these sites. The bacteriophage recombinase Cre catalyses site-specific recombination between two 34-base-pair loxP sites. The crystal structure at 2.4 A resolution of Cre bound to a loxP substrate reveals an intermediate in the recombination reaction, in which a Cre molecule has cleaved the substrate to form a covalent 3'-phosphotyrosine linkage with the DNA. Four recombinases and two loxP sites form a synapsed structure in which the DNA resembles models of four-way Holliday-Junction intermediates. The Cre-loxP complex challenges models of site-specific recombination that require large changes in quaternary structure. Subtle allosteric changes at the carboxy termini of the Cre subunits may instead coordinate the cleavage and strand-exchange reactions.