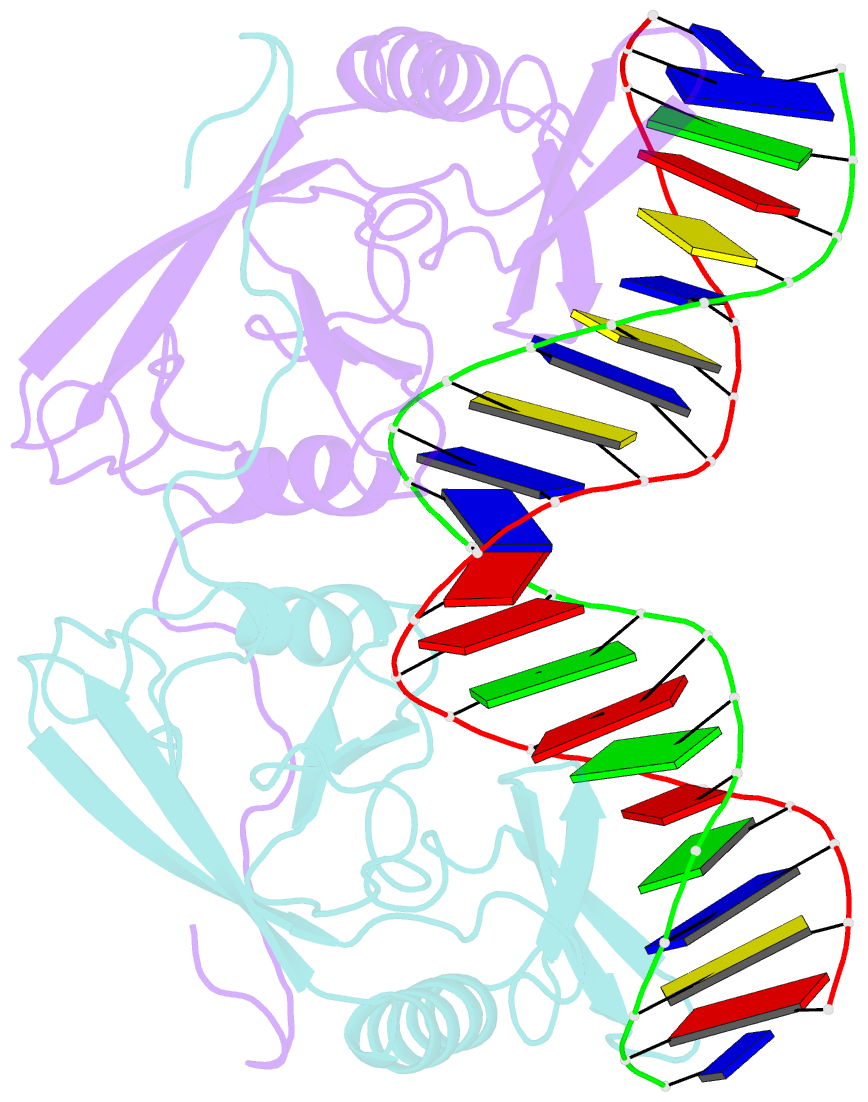
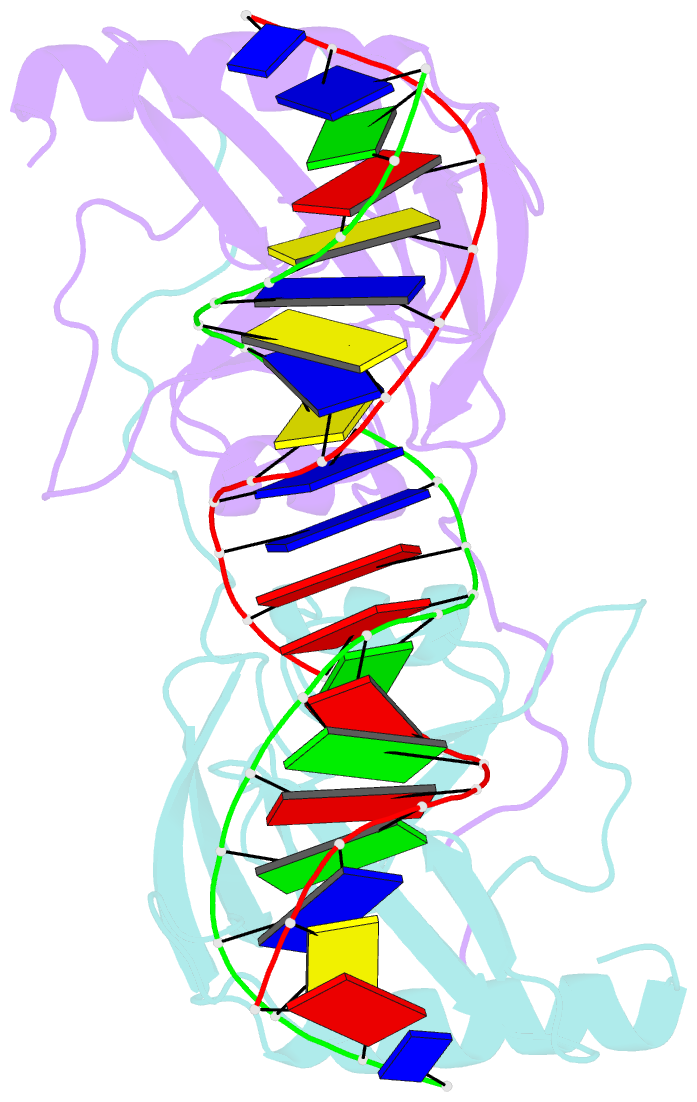
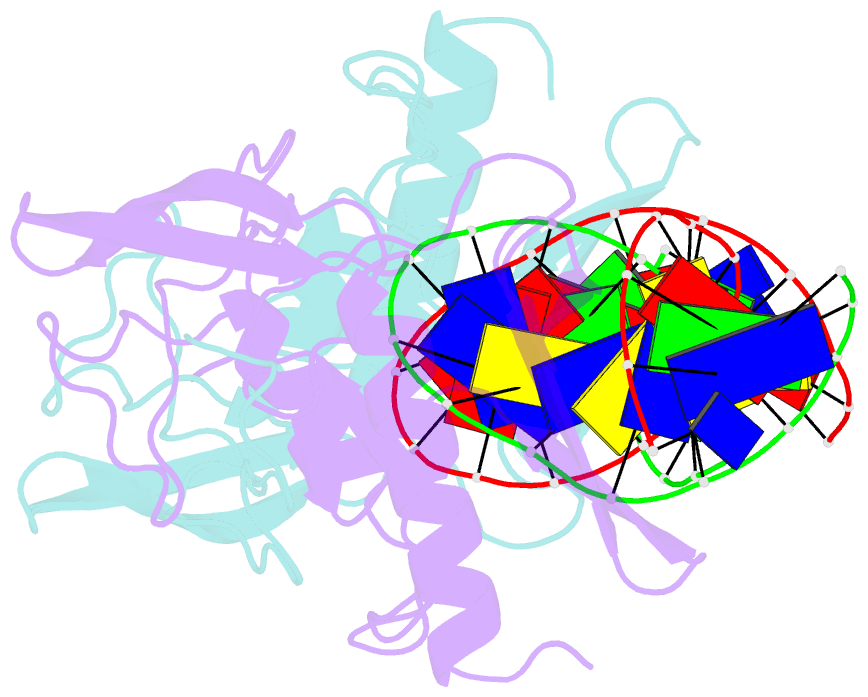
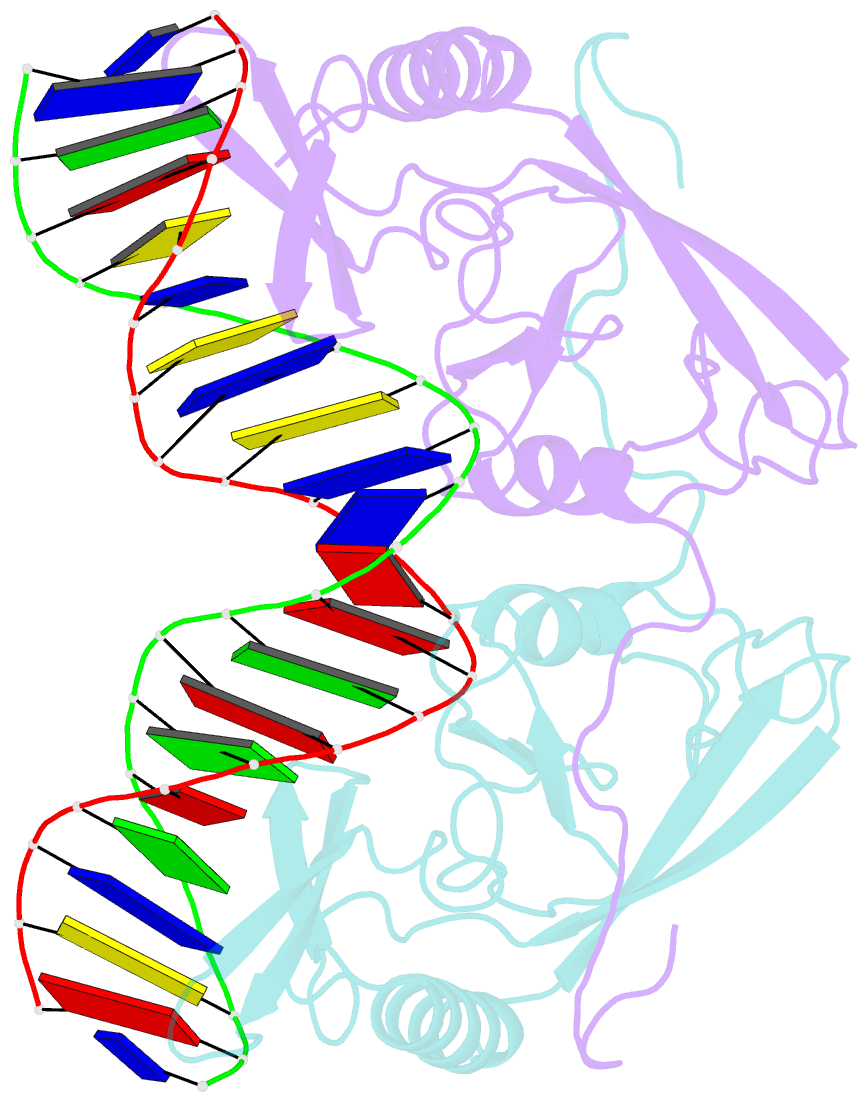
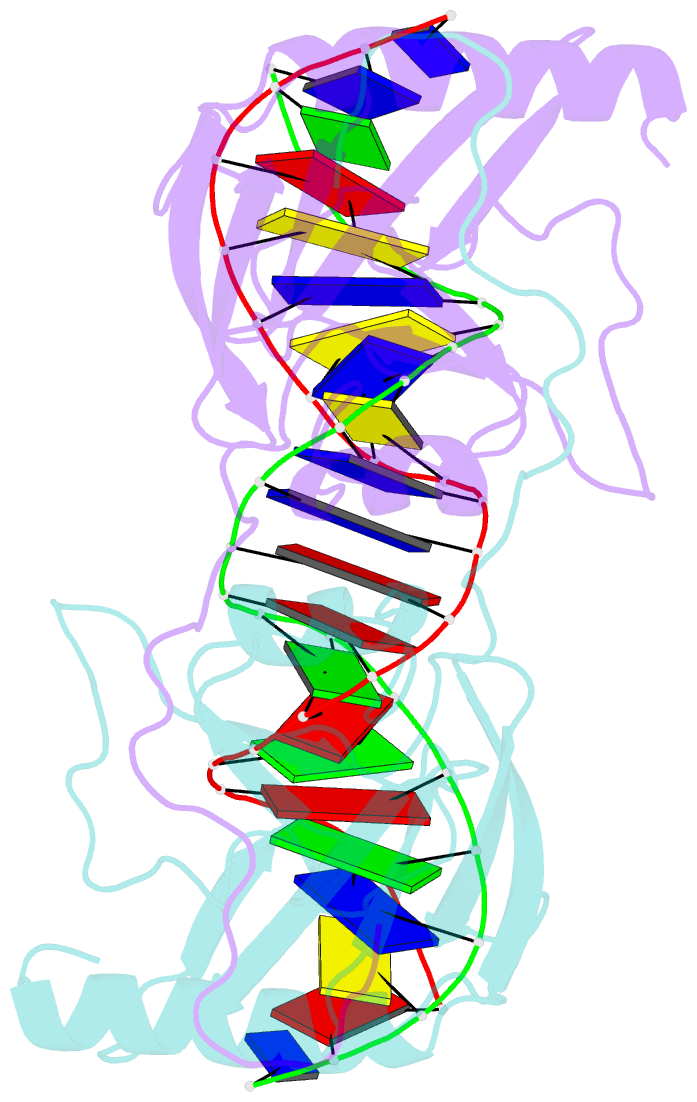
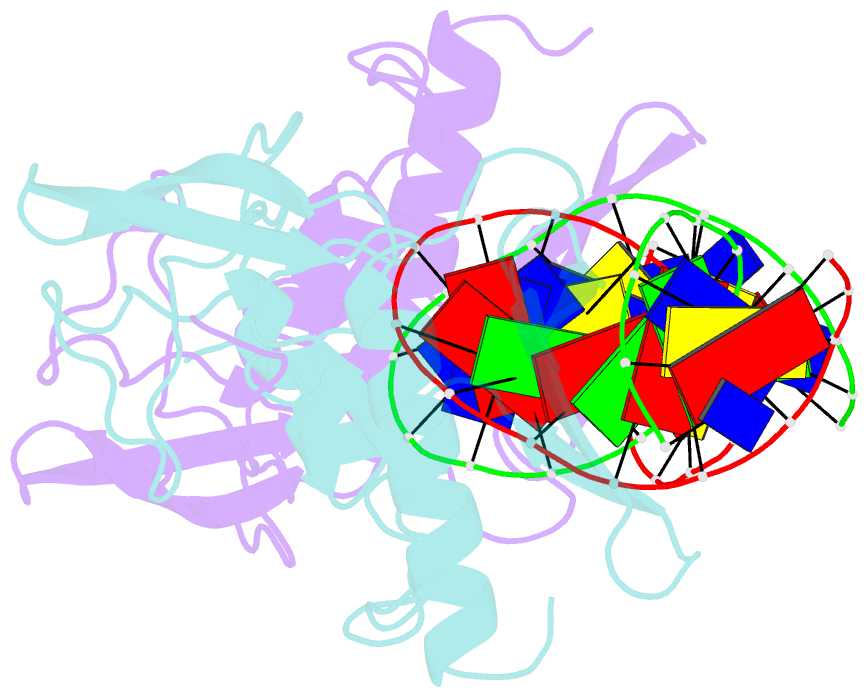
Summary information and primary citation
- PDB-id
- 1cz0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.1 Å)
- Summary
- Intron encoded homing endonuclease i-ppoi-DNA complex lacking catalytic metal ion
- Reference
- Galburt EA, Chevalier B, Tang W, Jurica MS, Flick KE, Monnat Jr RJ, Stoddard BL (1999): "A novel endonuclease mechanism directly visualized for I-PpoI." Nat.Struct.Biol., 6, 1096-1099. doi: 10.1038/70027.
- Abstract
- A novel mechanism of DNA endonucleolytic cleavage has been visualized for the homing endonuclease I-PpoI by trapping the uncleaved enzyme-substrate complex and comparing it to the previously visualized product complex. This enzyme employs a unique single metal mechanism. A magnesium ion is coordinated by an asparagine residue and two DNA oxygen atoms and stabilizes the phosphoanion transition state and the 3'oxygen leaving group. A hydrolytic water molecule is activated by a histidine residue for an in-line attack on the scissile phosphate. A strained enzyme-substrate-metal complex is formed before cleavage, then relaxed during the reaction.