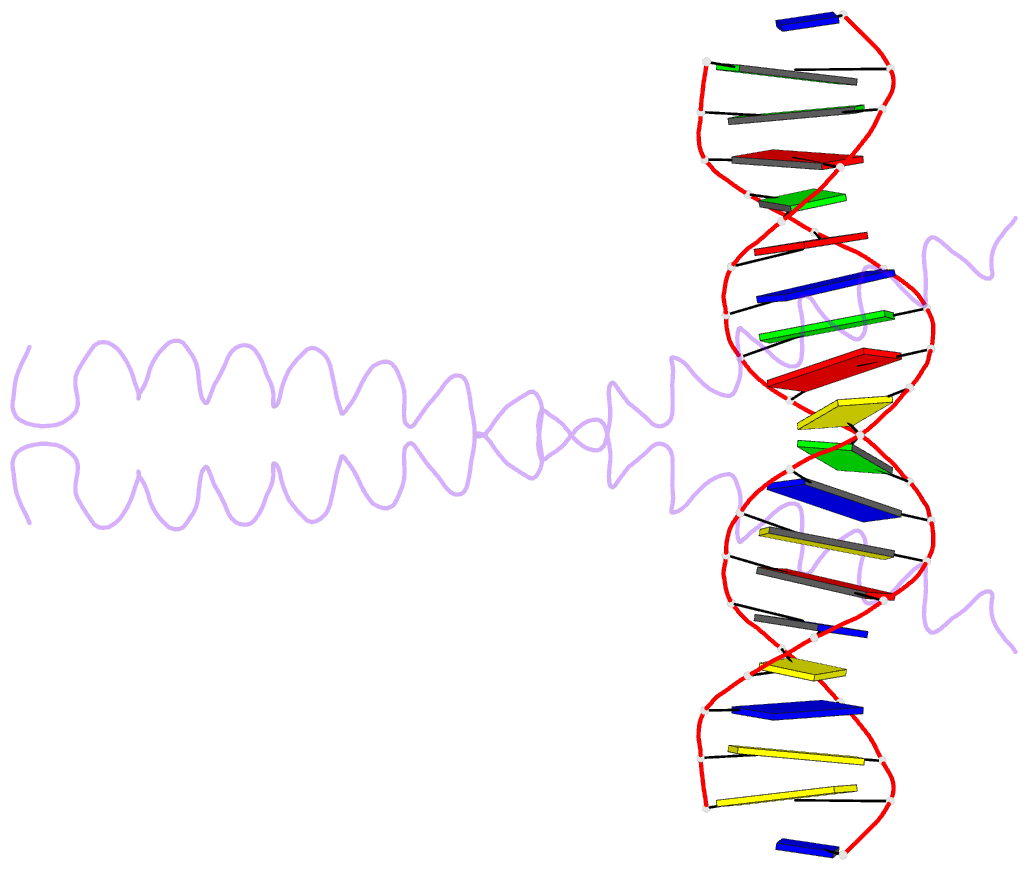
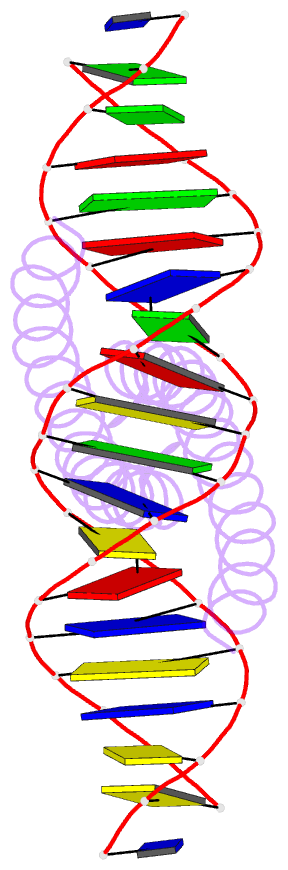
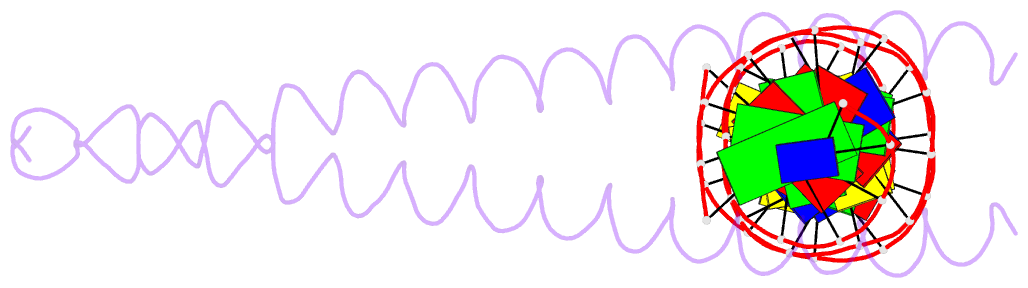
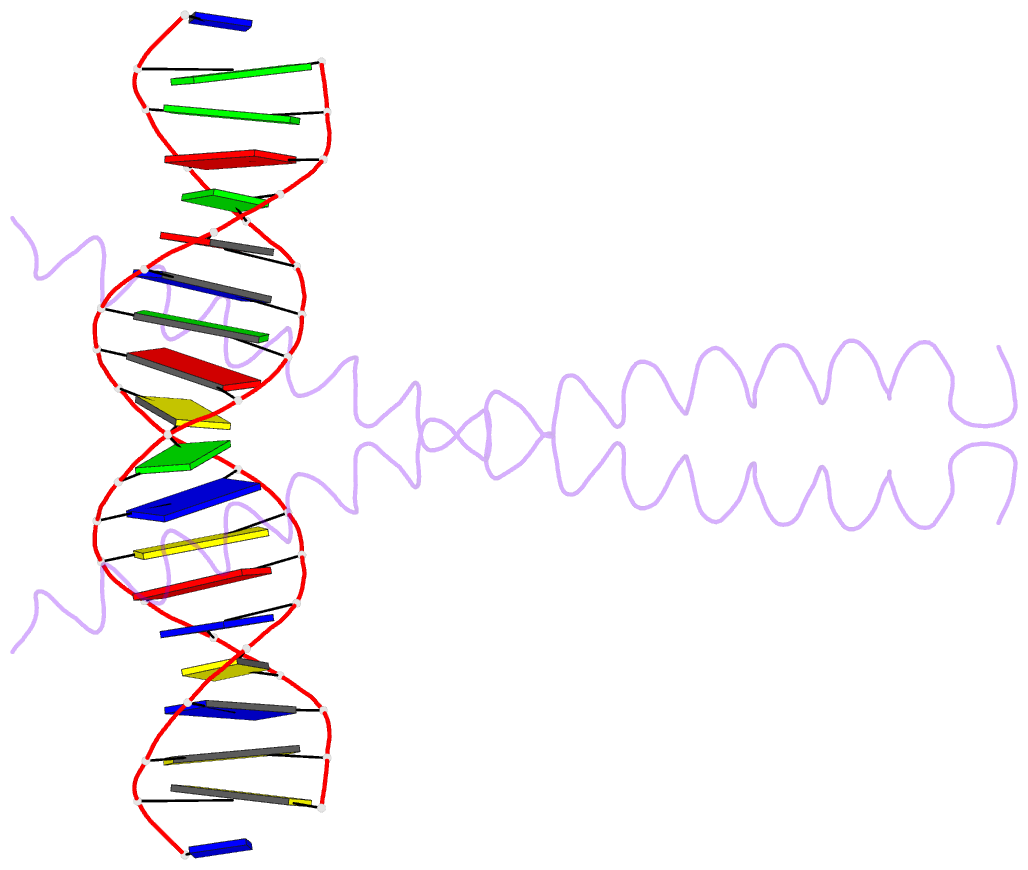
Summary information and primary citation
- PDB-id
- 1dgc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.0 Å)
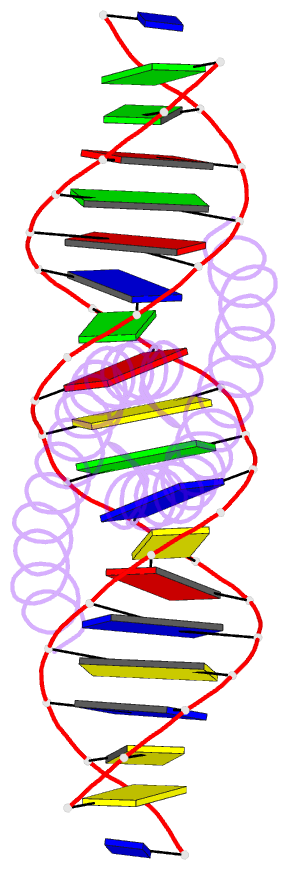
- Summary
- The x-ray structure of the gcn4-bzip bound to atf-creb site DNA shows the complex depends on DNA flexibility
- Reference
- Konig P, Richmond TJ (1993): "The X-ray structure of the GCN4-bZIP bound to ATF/CREB site DNA shows the complex depends on DNA flexibility." J.Mol.Biol., 233, 139-154. doi: 10.1006/jmbi.1993.1490.
- Abstract
- The X-ray structure of the DNA binding domain of the yeast transcriptional activator protein GCN4 bound to a DNA fragment containing the sequence of the perfectly symmetrical ATF/CREB site has been solved to 3.0 A resolution. The architecture of this specific recognition complex supports the current model for bZIP proteins: a homodimer of parallel alpha-helices form an interhelix coiled-coil region via the leucine zipper, and the two N-terminal basic regions fit into the major groove of half sites on opposite sides of the DNA double helix. The structure shows that DNA flexibility plays the predominant role in the preservation of protein contacts with the symmetric ATF/CREB site (ATGACGTCAT) as compared to the pseudo-symmetric AP-1 target site (ATGACTCAT), overcoming the positional displacement of functional groups introduced by the additional G.C base-pair at the center of the ATF/CREB sequence.