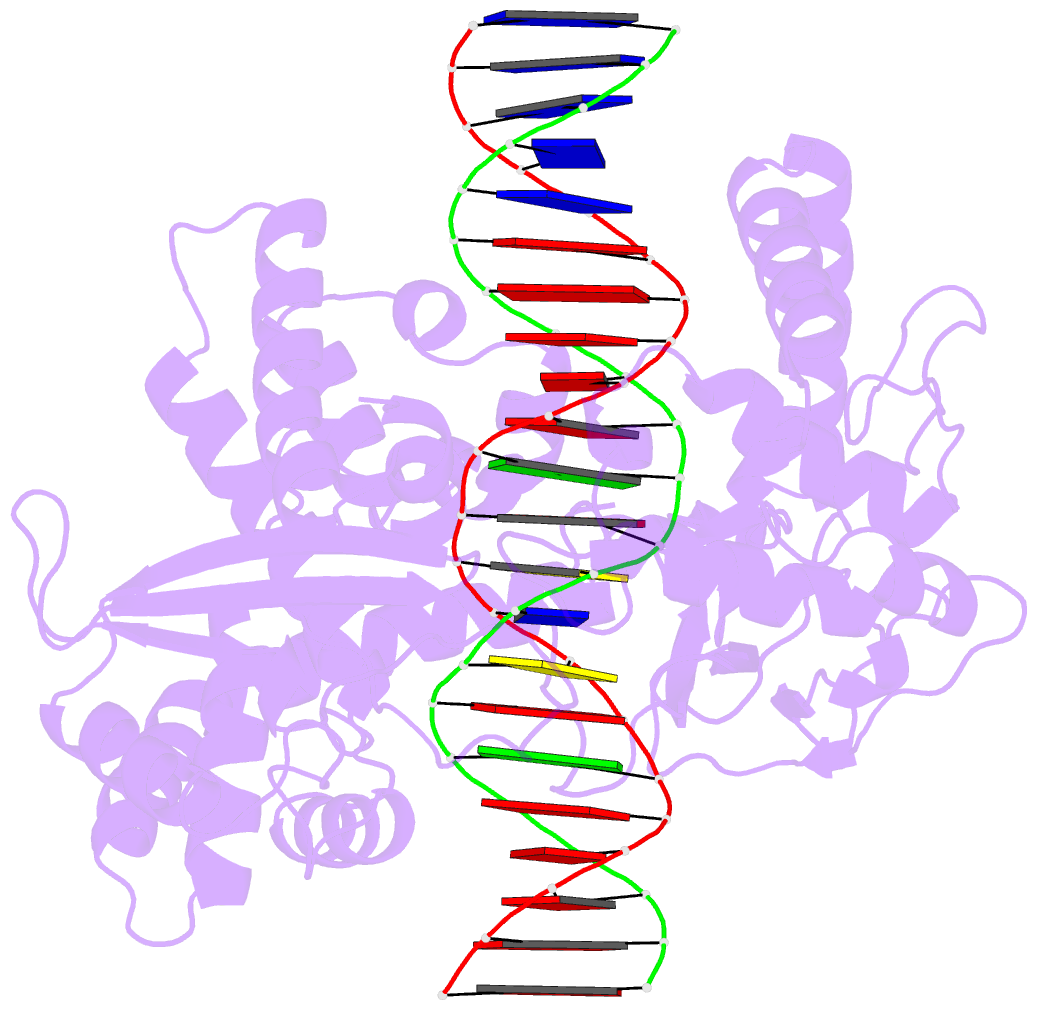
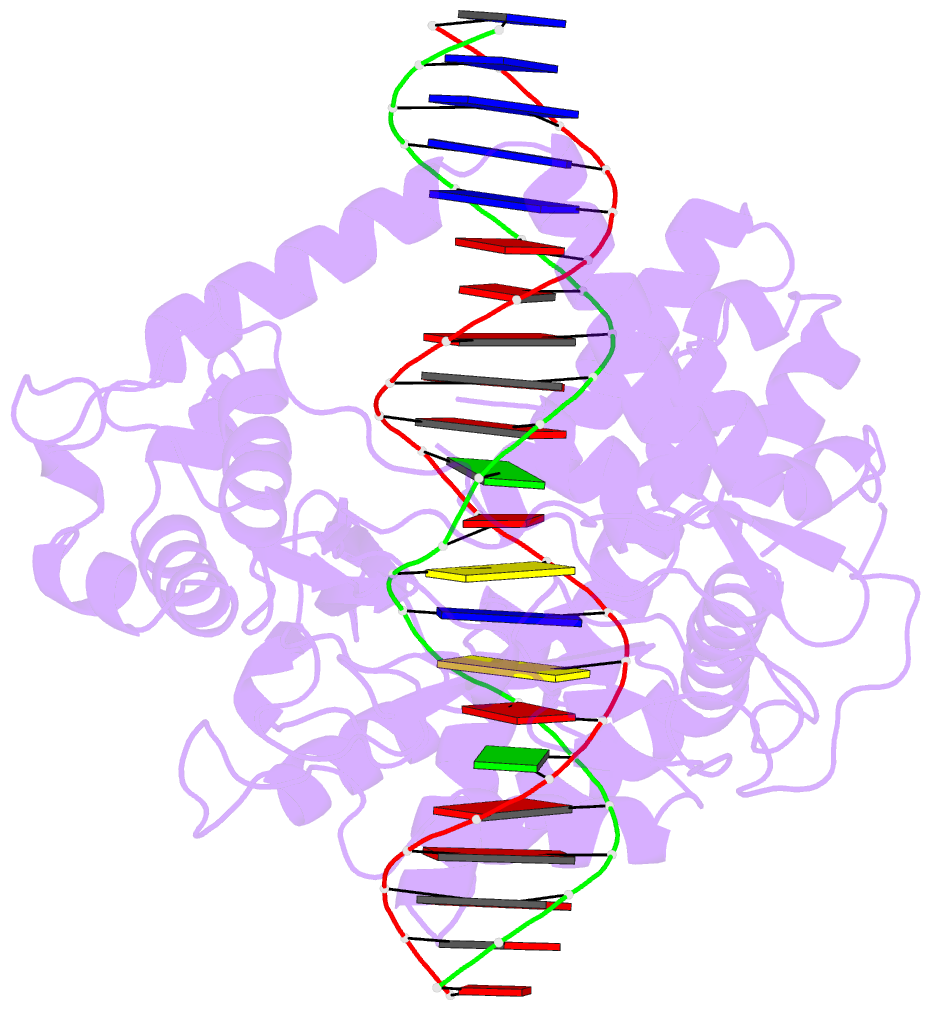
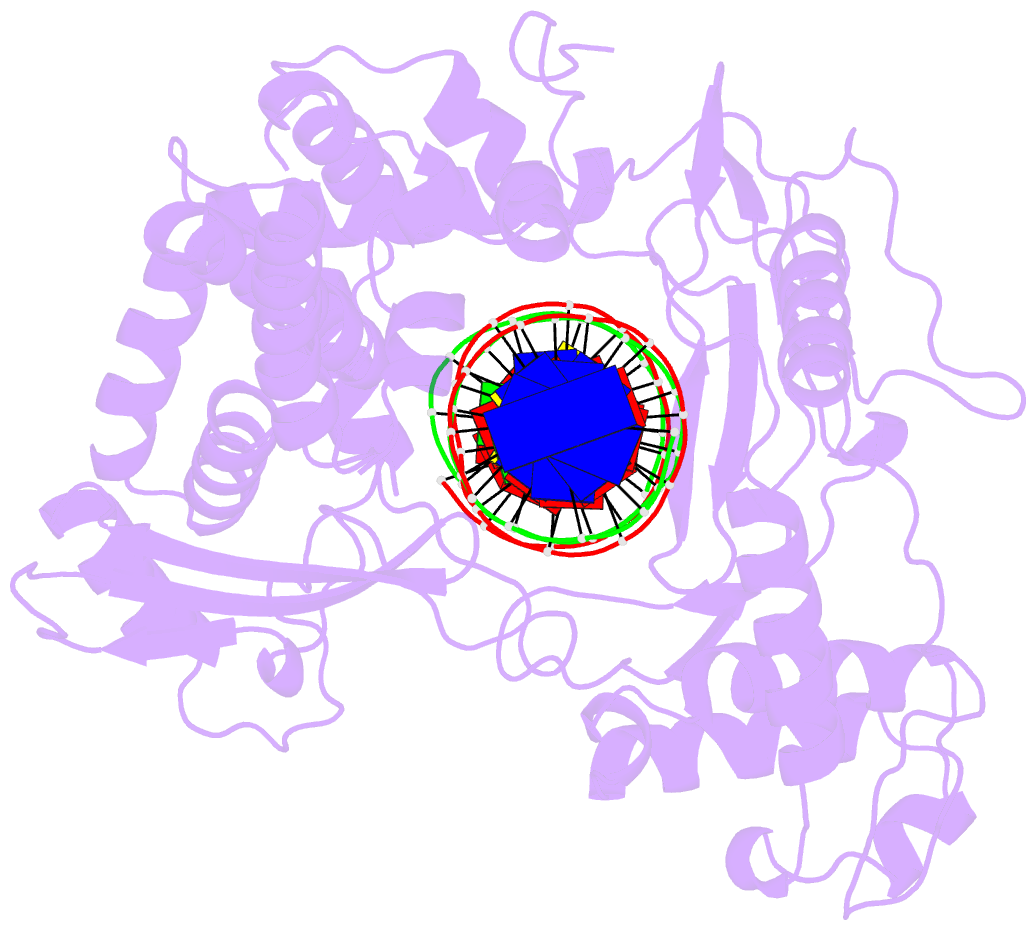
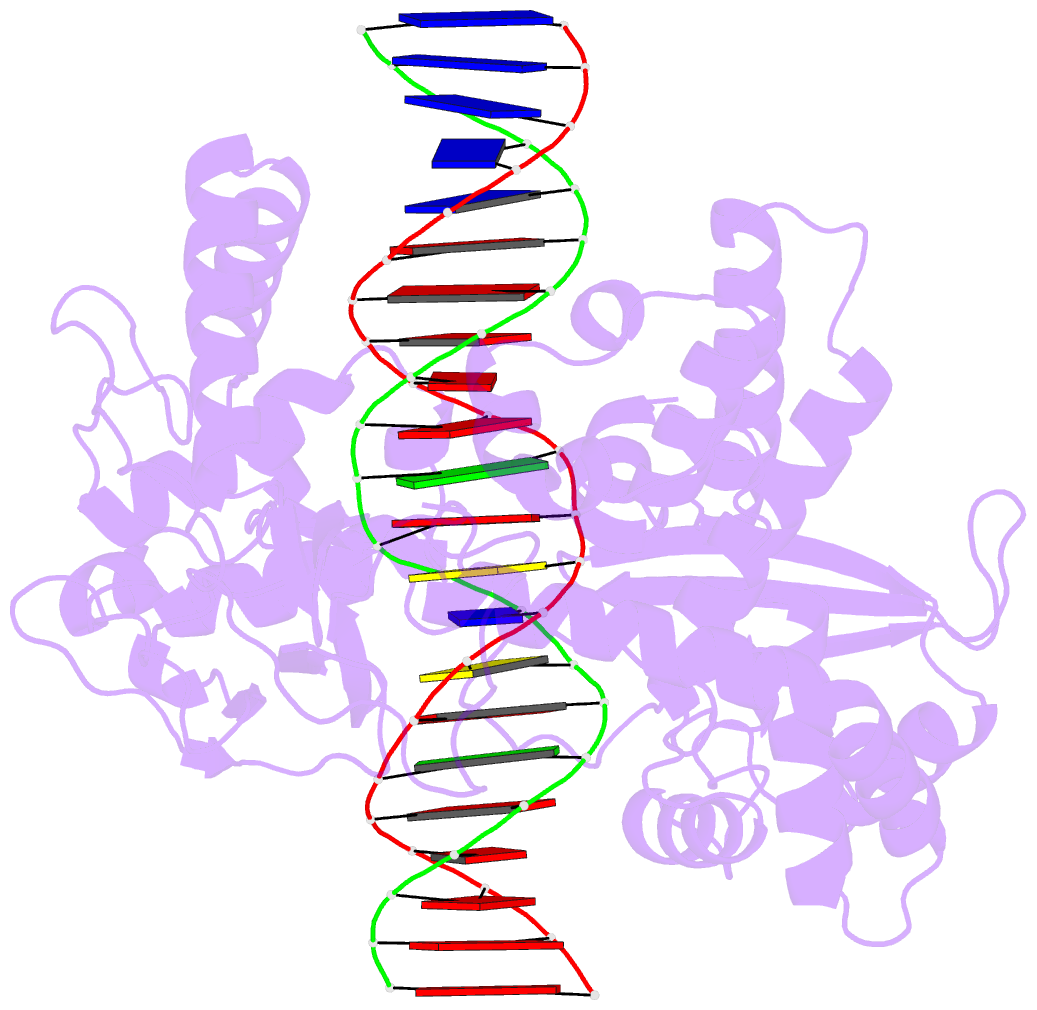
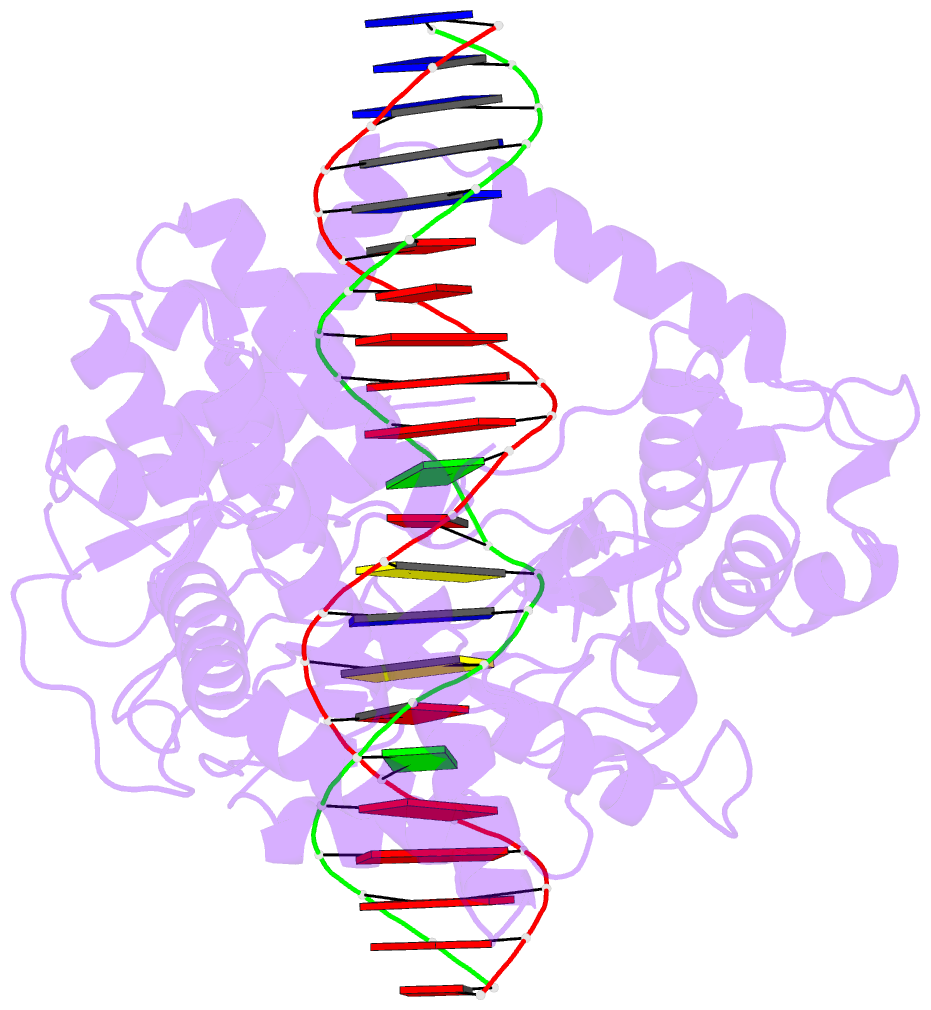
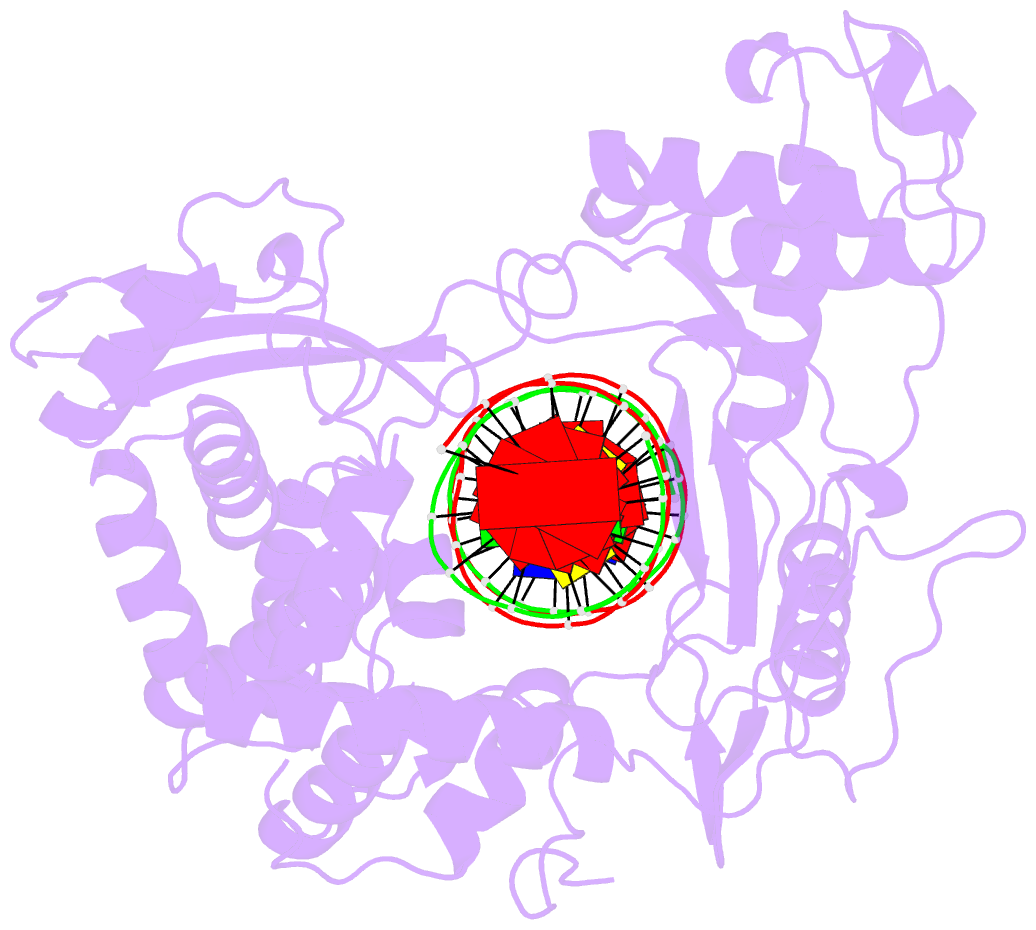
Summary information and primary citation
- PDB-id
- 1ej9; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA
- Method
- X-ray (2.6 Å)
- Summary
- Crystal structure of human topoisomerase i DNA complex
- Reference
- Redinbo MR, Champoux JJ, Hol WG (2000): "Novel insights into catalytic mechanism from a crystal structure of human topoisomerase I in complex with DNA." Biochemistry, 39, 6832-6840. doi: 10.1021/bi992690t.
- Abstract
- Human topoisomerase I helps to control the level of DNA supercoiling in cells and is vital for numerous DNA metabolic events, including replication, transcription, and recombination. The 2.6 A crystal structure of human topoisomerase I in noncovalent complex with a DNA duplex containing a cytosine at the -1 position of the scissile strand rather than the favored thymine is reported. The hydrogen bond between the O2 position of this -1 base and the epsilon-amino of the conserved Lys-532 residue, the only base-specific contact observed previously in the human topoisomerase I-DNA interaction, is maintained in this complex. Several unique features of this structure, however, have implications for the DNA-binding and active-site mechanisms of the enzyme. First, the ends of the DNA duplex were observed to shift by up to 5.4 A perpendicular to the DNA helical axis relative to structures reported previously, suggesting a novel degree of plasticity in the interaction between human topoisomerase I and its DNA substrate. Second, 12 additional residues at the NH(2) terminus of the protein (Trp-203-Gly-214) could be built in this structure, and they were found to pack against the putative hinge region implicated in the clamping of the enzyme around duplex DNA. Third, a water molecule was observed adjacent to the scissile phosphate and the active-site residues; the potential specific base character of this solvent molecule in the active-site mechanism of the enzyme is discussed. Fourth, the scissile phosphate group was found to be rotated by 75 degrees, bringing Lys-532 into hydrogen-bonding distance of one of the nonbridging phosphate oxygens. This orientation of the scissile phosphate group implicates Lys-532 as a fifth active-site residue, and also mimics the orientation observed for the 3'-phosphotyrosine linkage in the covalent human topoisomerase I-DNA complex structure. The implications of these structural features for the mechanism of the enzyme are discussed, including the potential requirement for a rotation of the scissile phosphate group during DNA strand cleavage and covalent attachment.