Summary information and primary citation
- PDB-id
- 1f2i; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.35 Å)
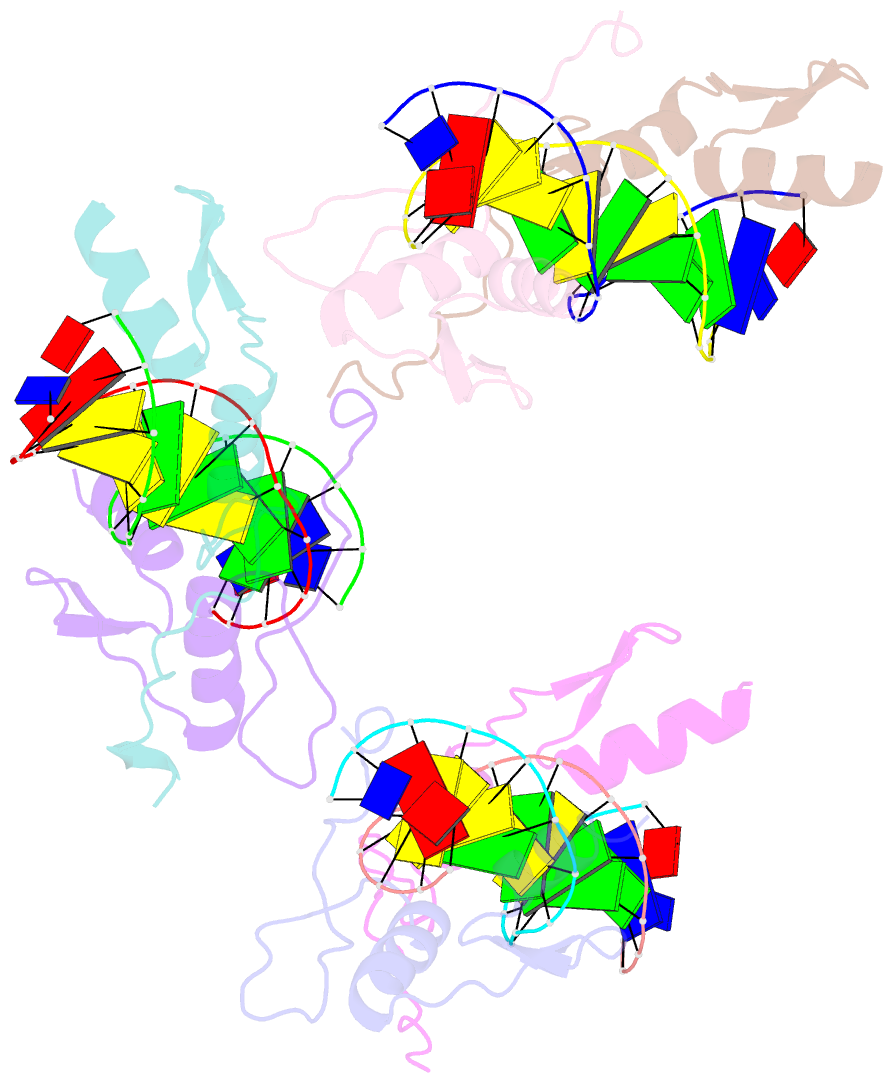
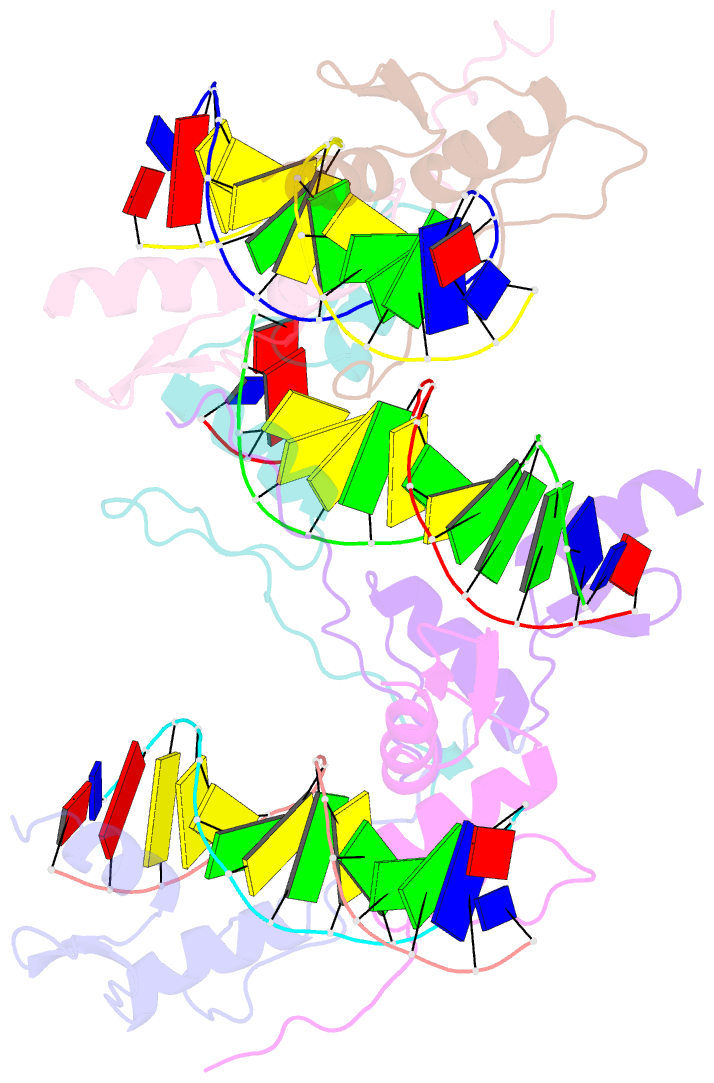
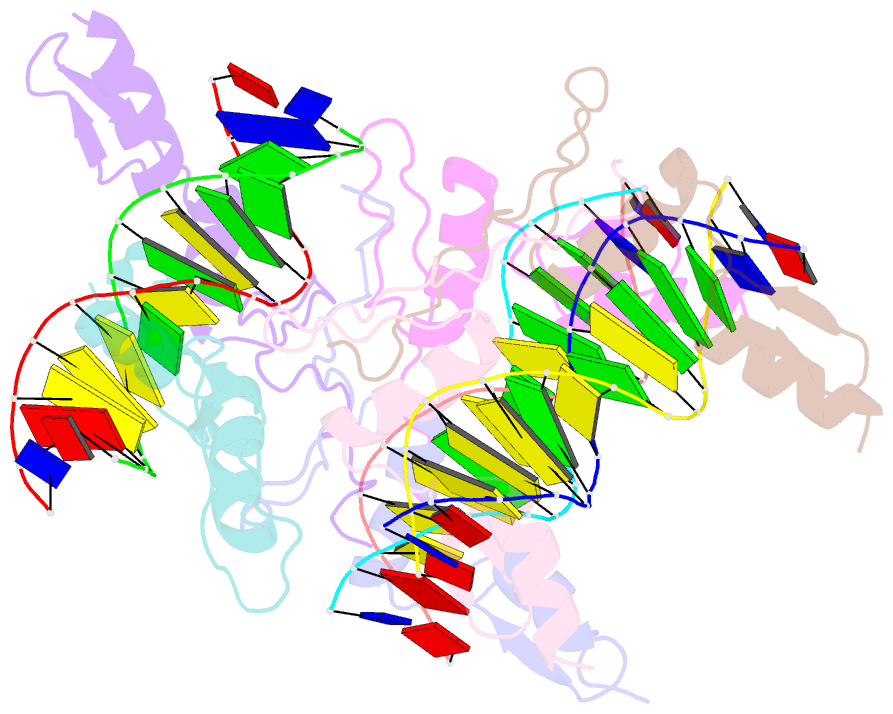
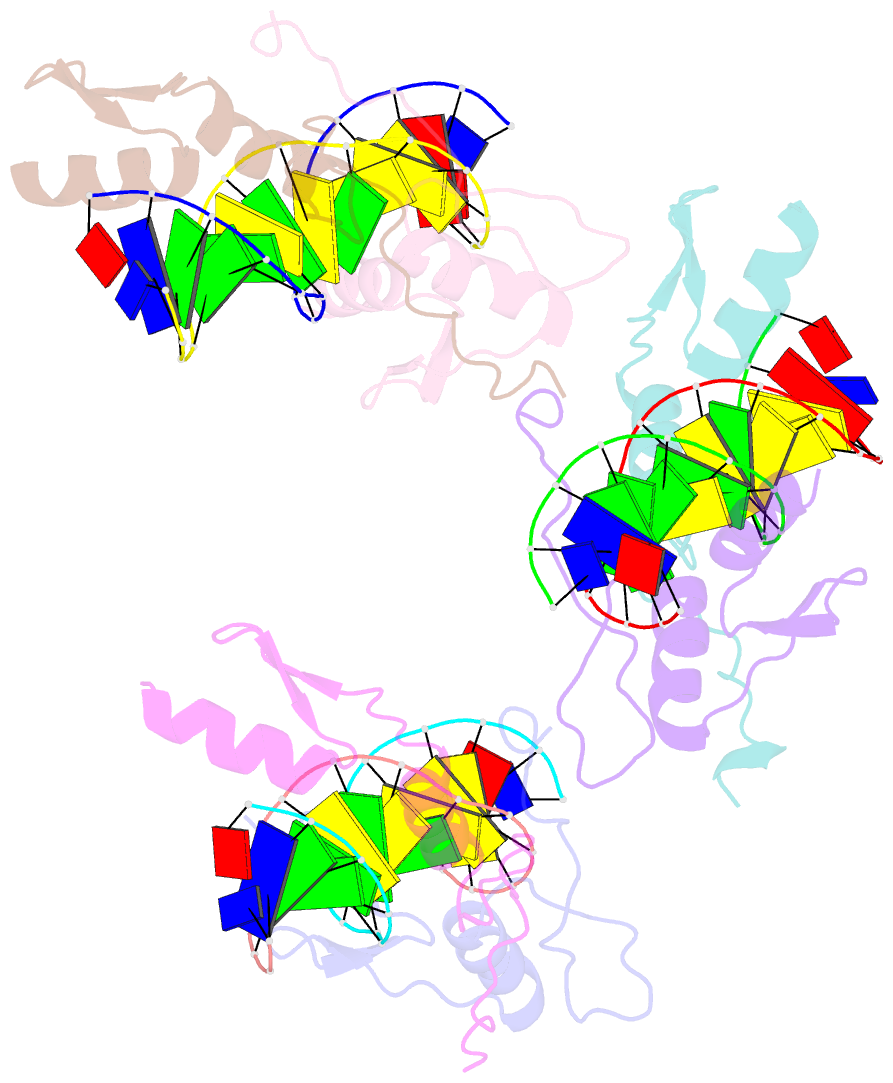
- Summary
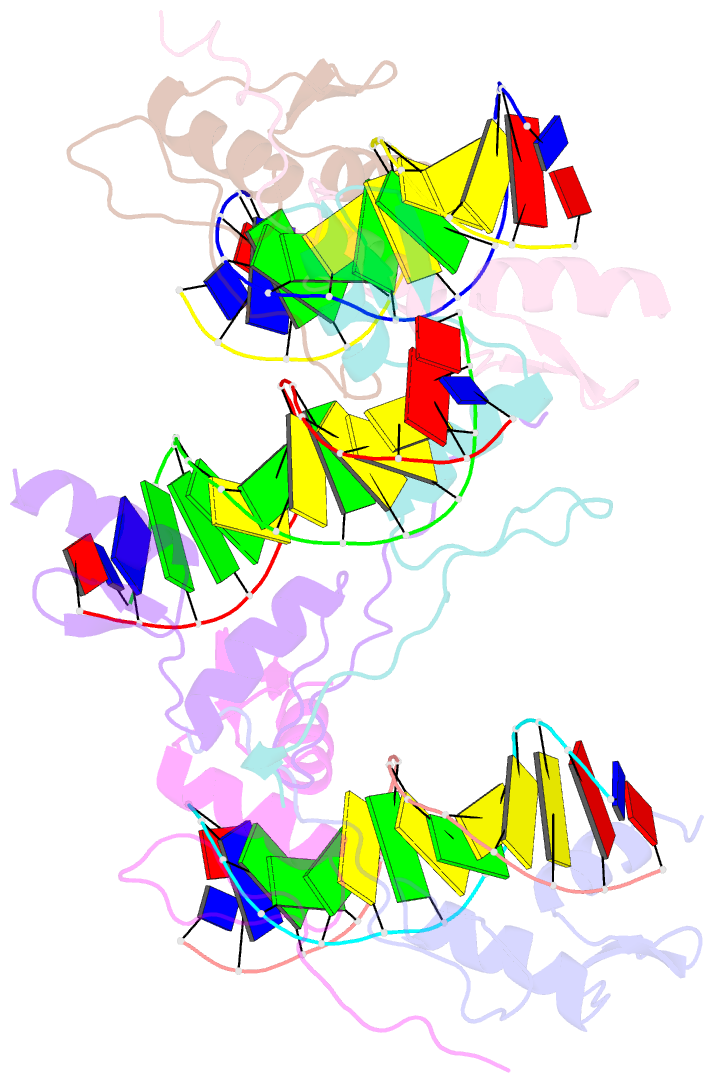
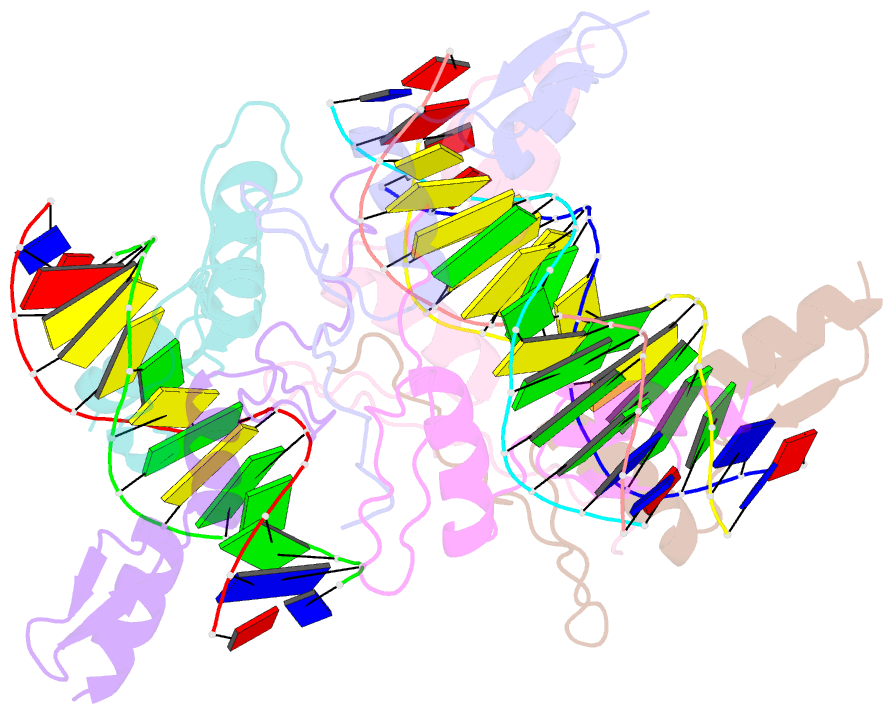
- Cocrystal structure of selected zinc finger dimer bound to DNA
- Reference
- Wang BS, Grant RA, Pabo CO (2001): "Selected peptide extension contacts hydrophobic patch on neighboring zinc finger and mediates dimerization on DNA." Nat.Struct.Biol., 8, 589-593. doi: 10.1038/89617.
- Abstract
- Protein-protein interactions often play a crucial role in stabilizing protein-DNA complexes and thus facilitate site-specific DNA recognition. We have worked to incorporate such protein-protein contacts into our design and selection strategies for short peptide extensions that promote cooperative binding of zinc finger proteins to DNA. We have determined the crystal structure of one of these fusion protein-DNA complexes. The selected peptide extension was found to mediate dimerization by reaching across the dyad axis and contacting a hydrophobic patch on the surface of the zinc finger bound to the adjacent DNA site. The peptide-zinc finger protein interactions observed in this structure are similar to those of some homeodomain heterodimers. We also find that the region of the zinc finger surface contacted by the selected peptide extension corresponds to surfaces that also make key interactions in the zinc finger proteins GLI and SWI5.