Summary information and primary citation
- PDB-id
- 1f4k; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication-DNA
- Method
- X-ray (2.5 Å)
- Summary
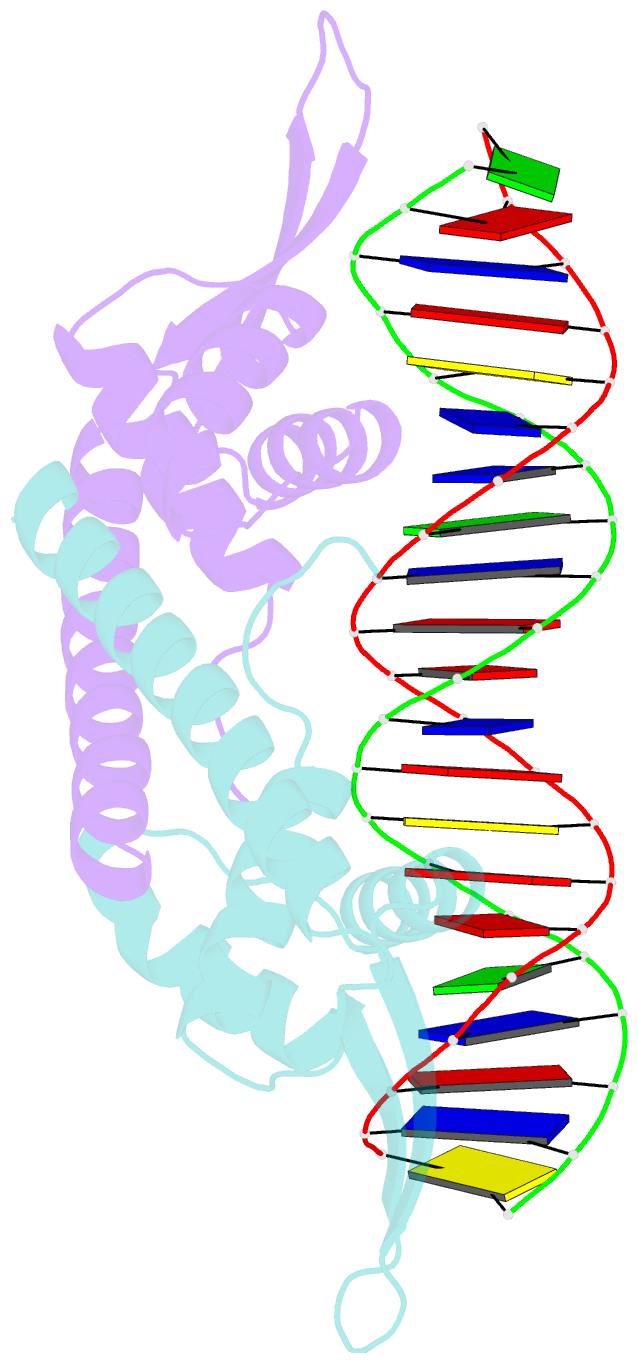
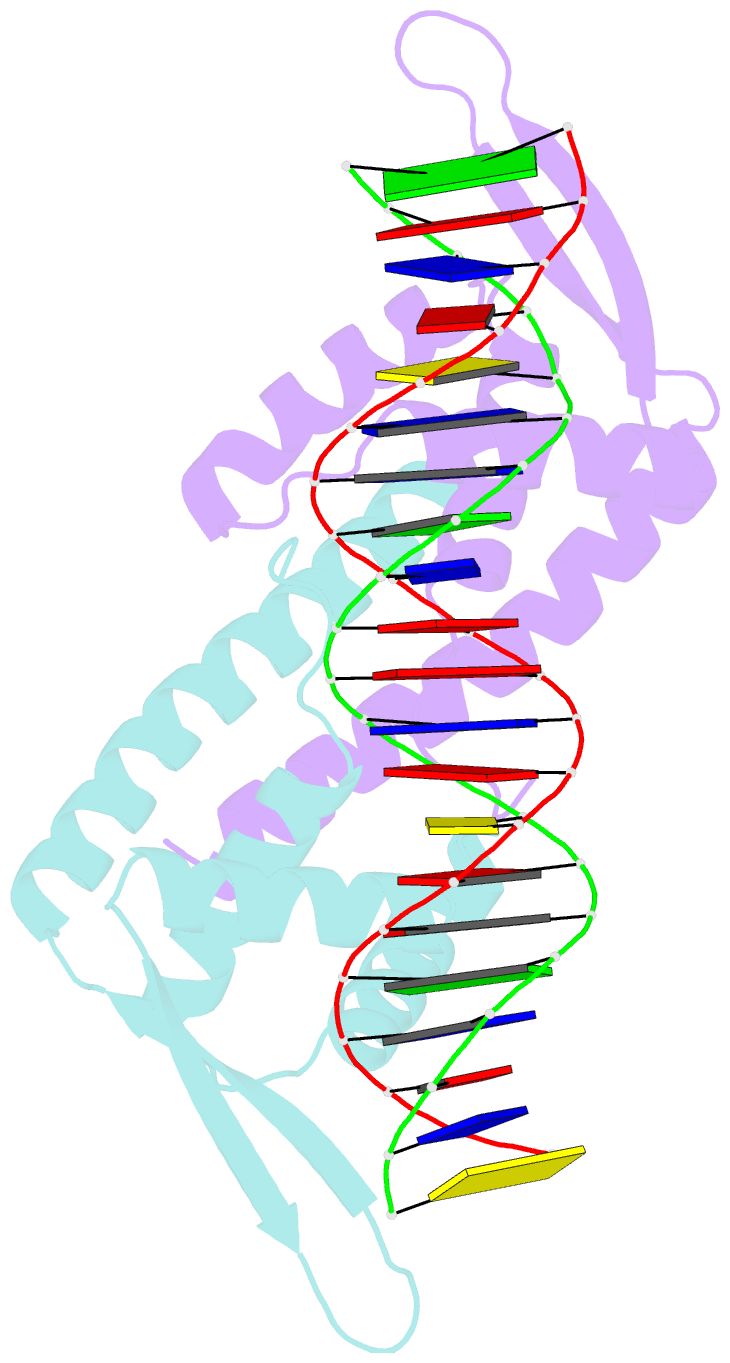
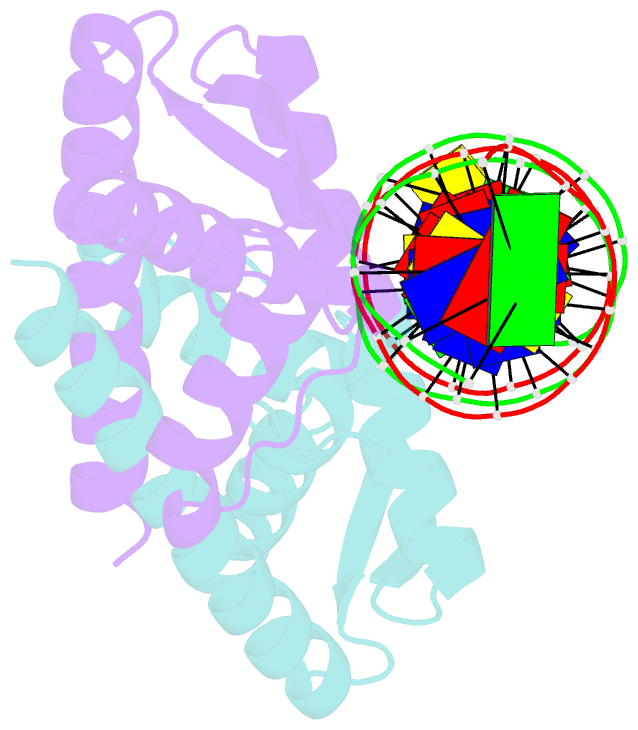
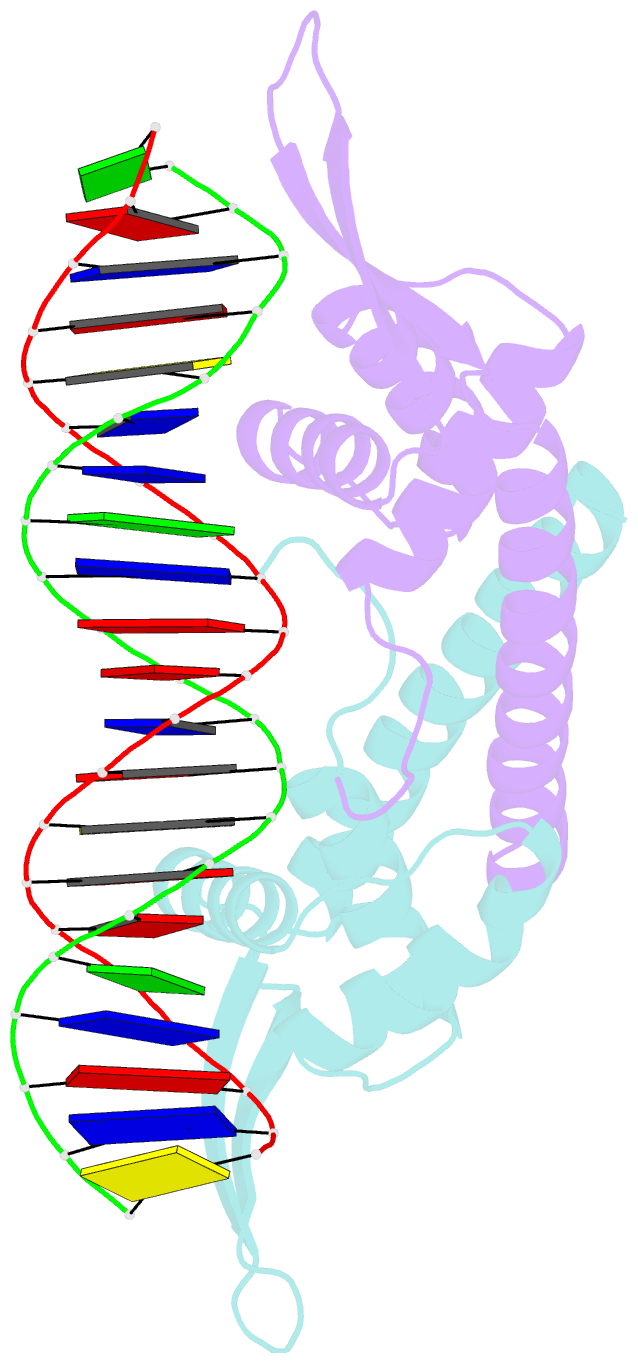
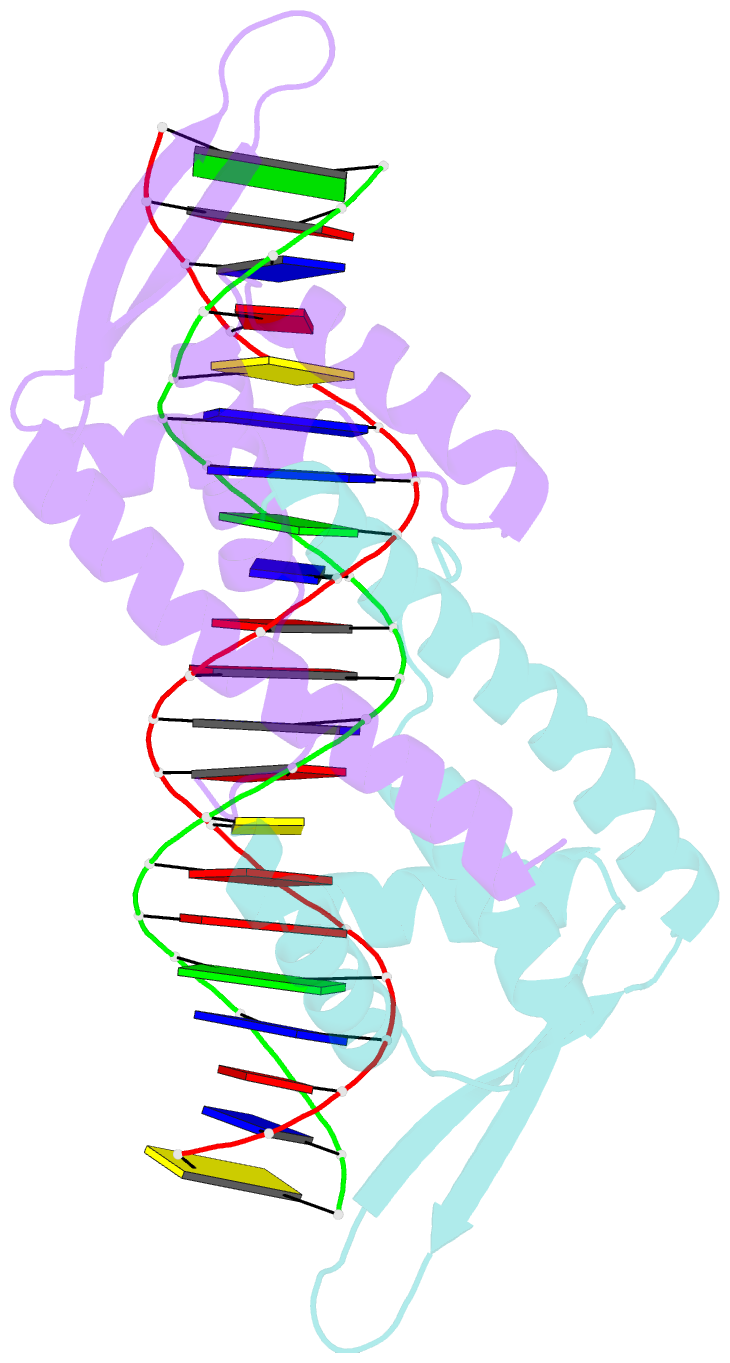
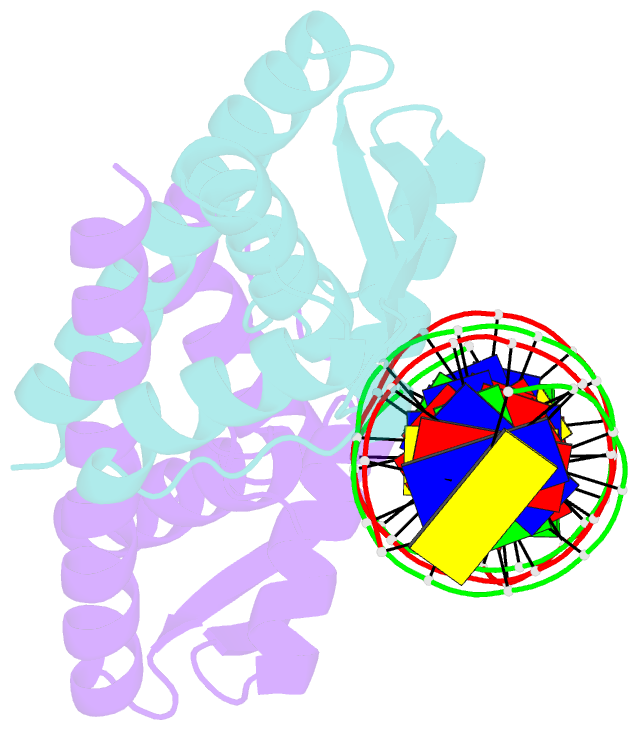
- Crystal structure of the replication terminator protein-b-site DNA complex
- Reference
- Wilce JA, Vivian JP, Hastings AF, Otting G, Folmer RH, Duggin IG, Wake RG, Wilce MC (2001): "Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest." Nat.Struct.Biol., 8, 206-210. doi: 10.1038/84934.
- Abstract
- The coordinated termination of DNA replication is an important step in the life cycle of bacteria with circular chromosomes, but has only been defined at a molecular level in two systems to date. Here we report the structure of an engineered replication terminator protein (RTP) of Bacillus subtilis in complex with a 21 base pair DNA by X-ray crystallography at 2.5 A resolution. We also use NMR spectroscopic titration techniques. This work reveals a novel DNA interaction involving a dimeric 'winged helix' domain protein that differs from predictions. While the two recognition helices of RTP are in close contact with the B-form DNA major grooves, the 'wings' and N-termini of RTP do not form intimate contacts with the DNA. This structure provides insight into the molecular basis of polar replication fork arrest based on a model of cooperative binding and differential binding affinities of RTP to the two adjacent binding sites in the complete terminator.