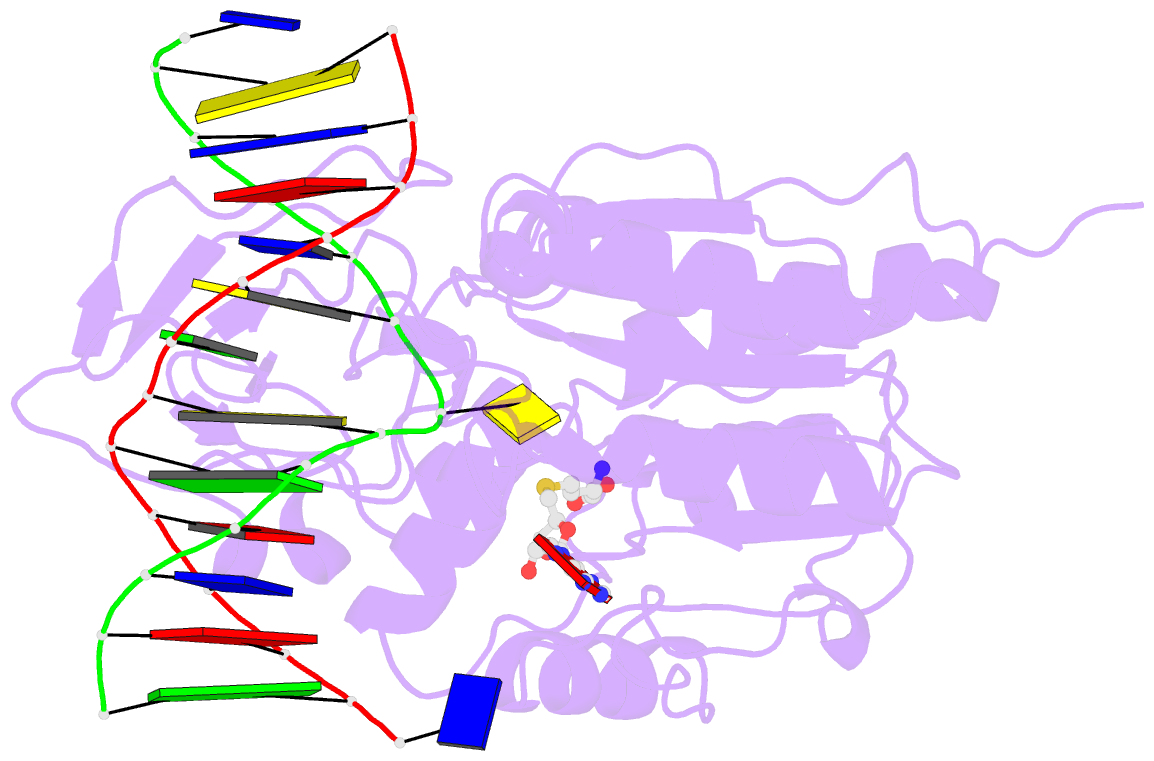
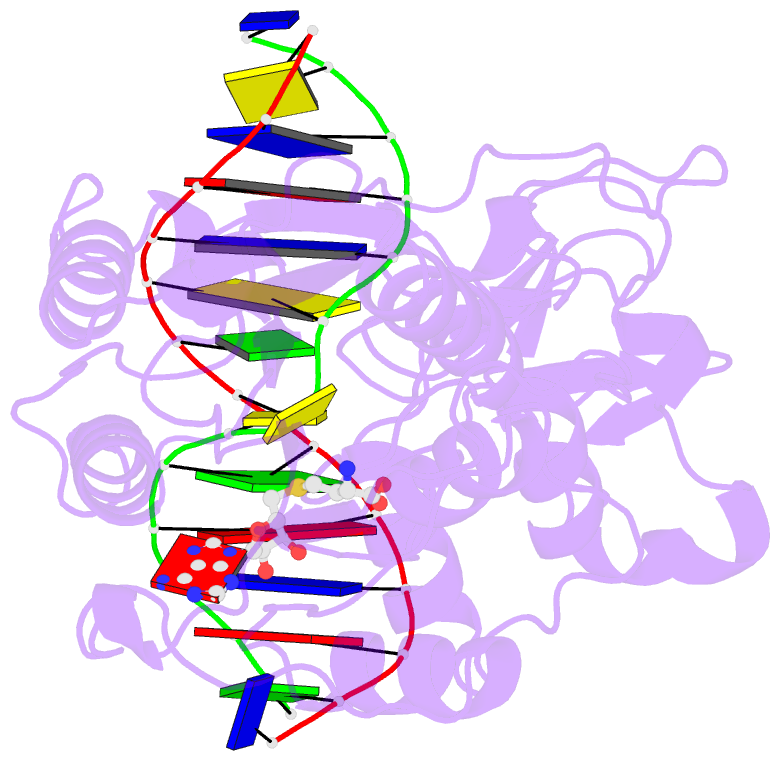
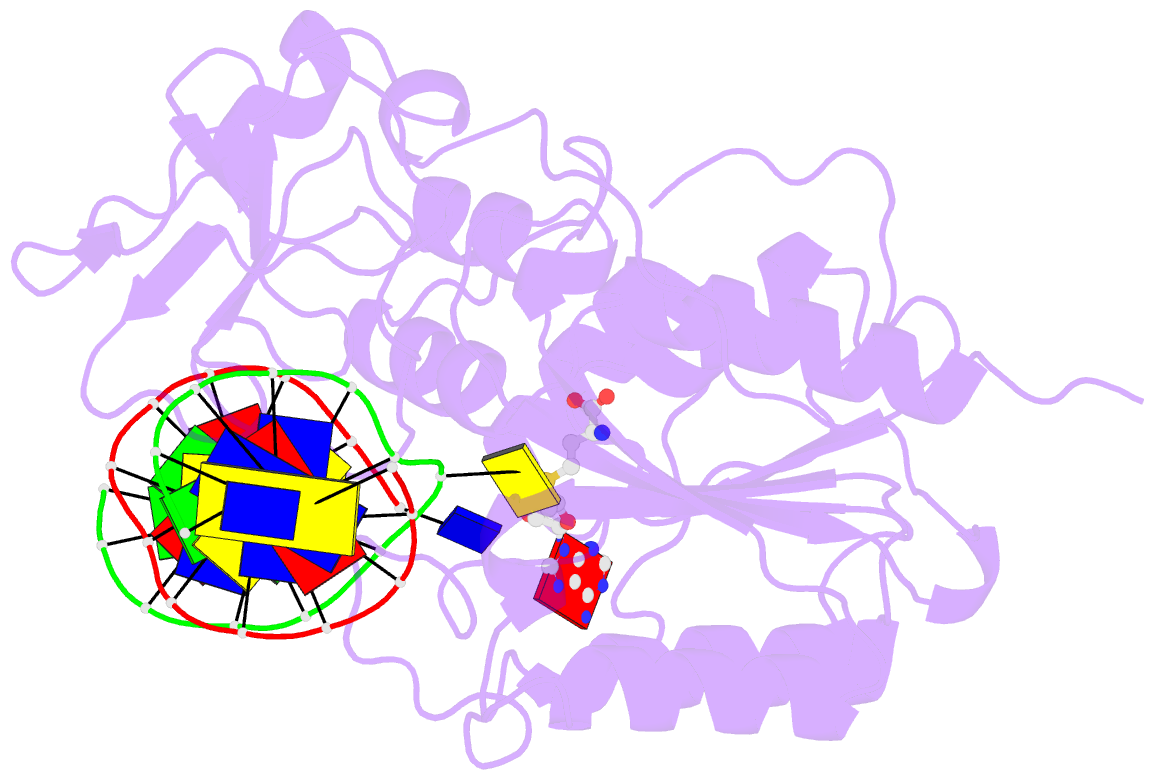
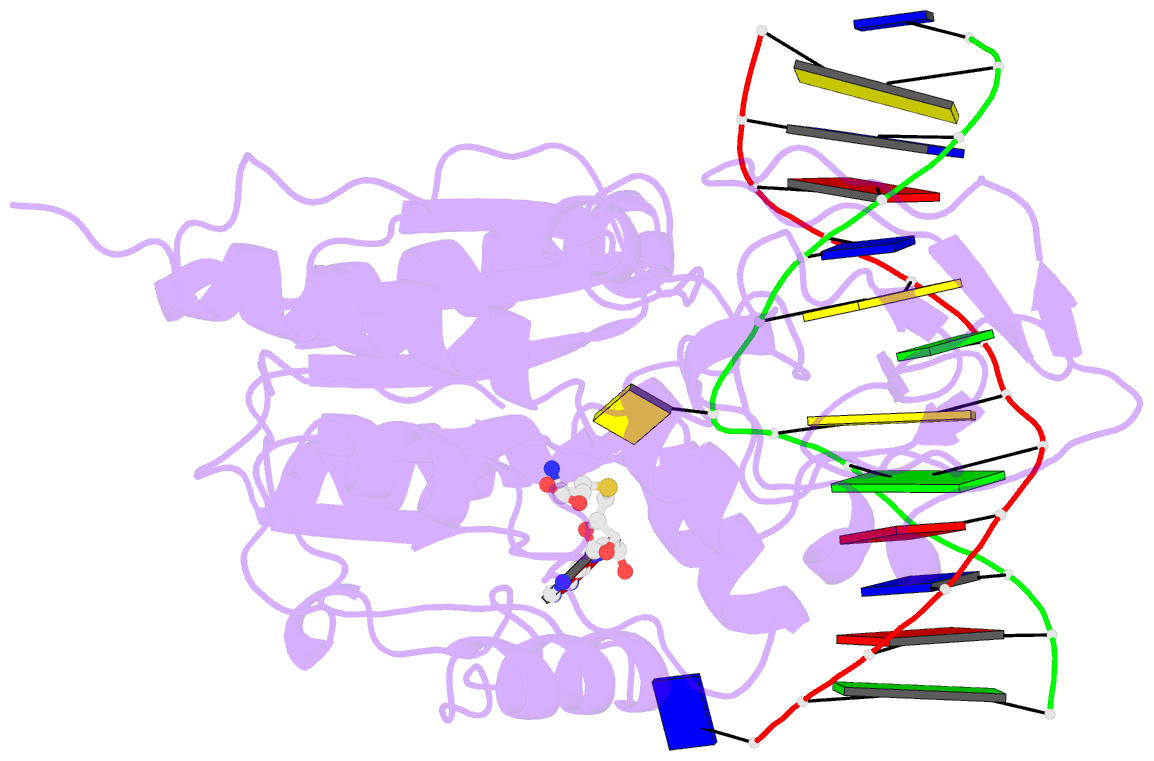
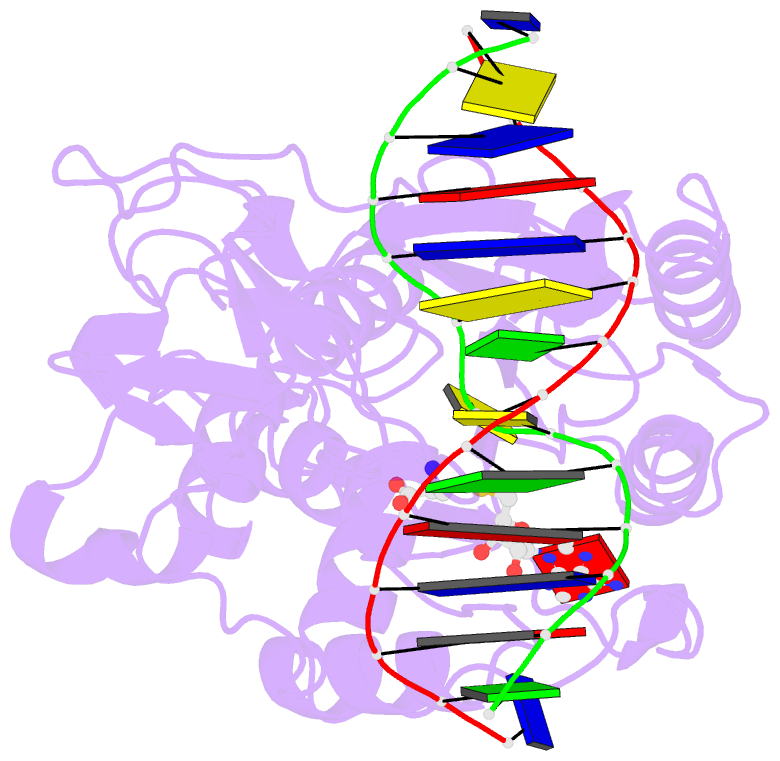
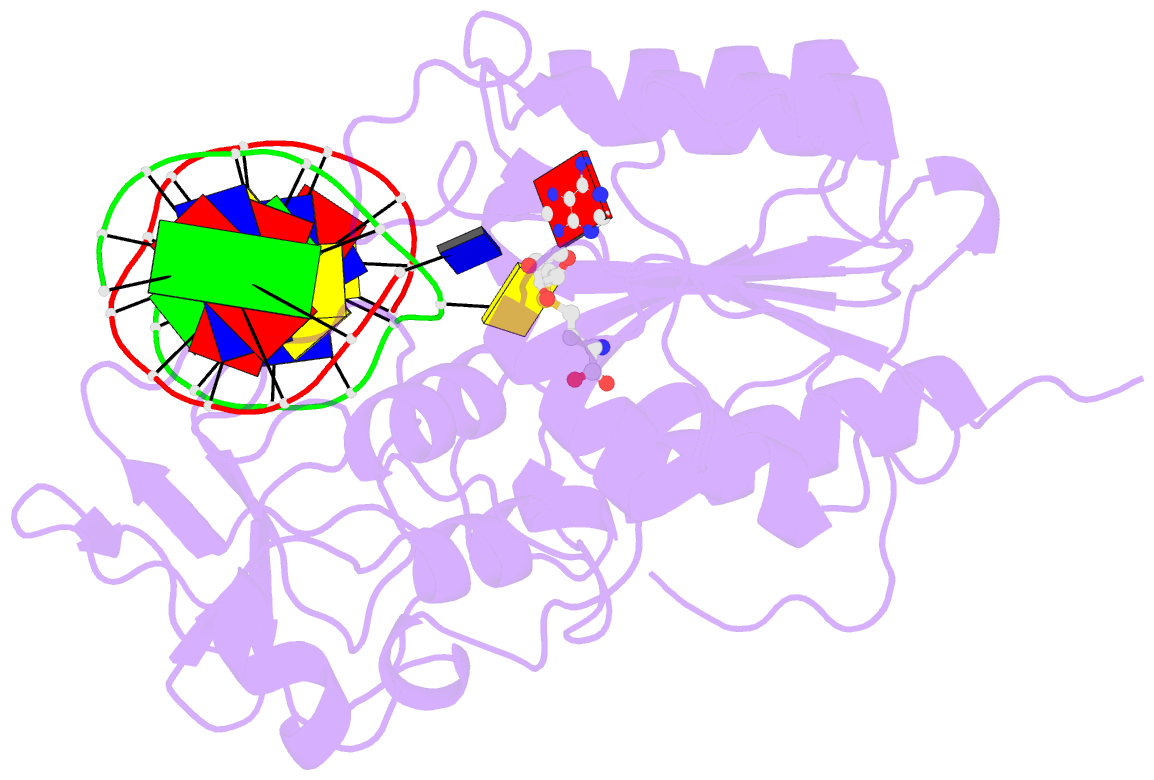
Summary information and primary citation
- PDB-id
- 1fjx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.26 Å)
- Summary
- Structure of ternary complex of hhai methyltransferase mutant (t250g) in complex with DNA and adohcy
- Reference
- Vilkaitis G, Dong A, Weinhold E, Cheng X, Klimasauskas S (2000): "Functional roles of the conserved threonine 250 in the target recognition domain of HhaI DNA methyltransferase." J.Biol.Chem., 275, 38722-38730.
- Abstract
- DNA cytosine-5-methyltransferase HhaI recognizes the GCGC sequence and flips the inner cytosine out of DNA helix and into the catalytic site for methylation. The 5'-phosphate of the flipped out cytosine is in contact with the conserved Thr-250 from the target recognition domain. We have produced 12 mutants of Thr-250 and examined their methylation potential in vivo. Six active mutants were subjected to detailed biochemical and structural studies. Mutants with similar or smaller side chains (Ser, Cys, and Gly) are very similar to wild-type enzyme in terms of steady-state kinetic parameters k(cat), K(m)(DNA), K(m)(AdoMet). In contrast, the mutants with bulkier side chains (Asn, Asp, and His) show increased K(m) values for both substrates. Fluorescence titrations and stopped-flow kinetic analysis of interactions with duplex oligonucleotides containing 2-aminopurine at the target base position indicate that the T250G mutation leads to a more polar but less solvent-accessible position of the flipped out target base. The x-ray structure of the ternary M. HhaI(T250G).DNA.AdoHcy complex shows that the target cytosine is locked in the catalytic center of enzyme. The space created by the mutation is filled by water molecules and the adjacent DNA backbone atoms dislocate slightly toward the missing side chain. In aggregate, our results suggest that the side chain of Thr-250 is involved in constraining the conformation the DNA backbone and the target base during its rotation into the catalytic site of enzyme.