Summary information and primary citation
- PDB-id
- 1fw6; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- replication-DNA
- Method
- X-ray (2.7 Å)
- Summary
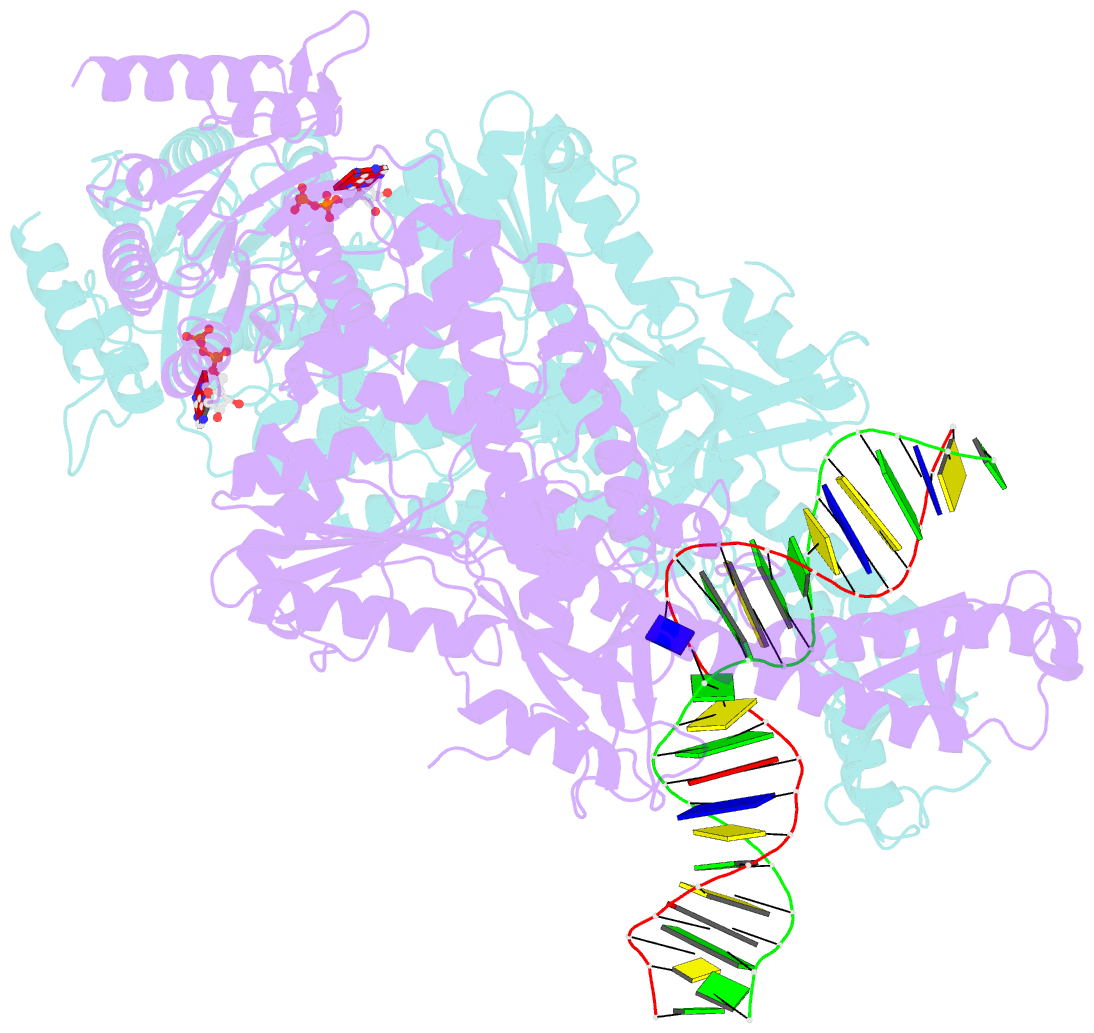
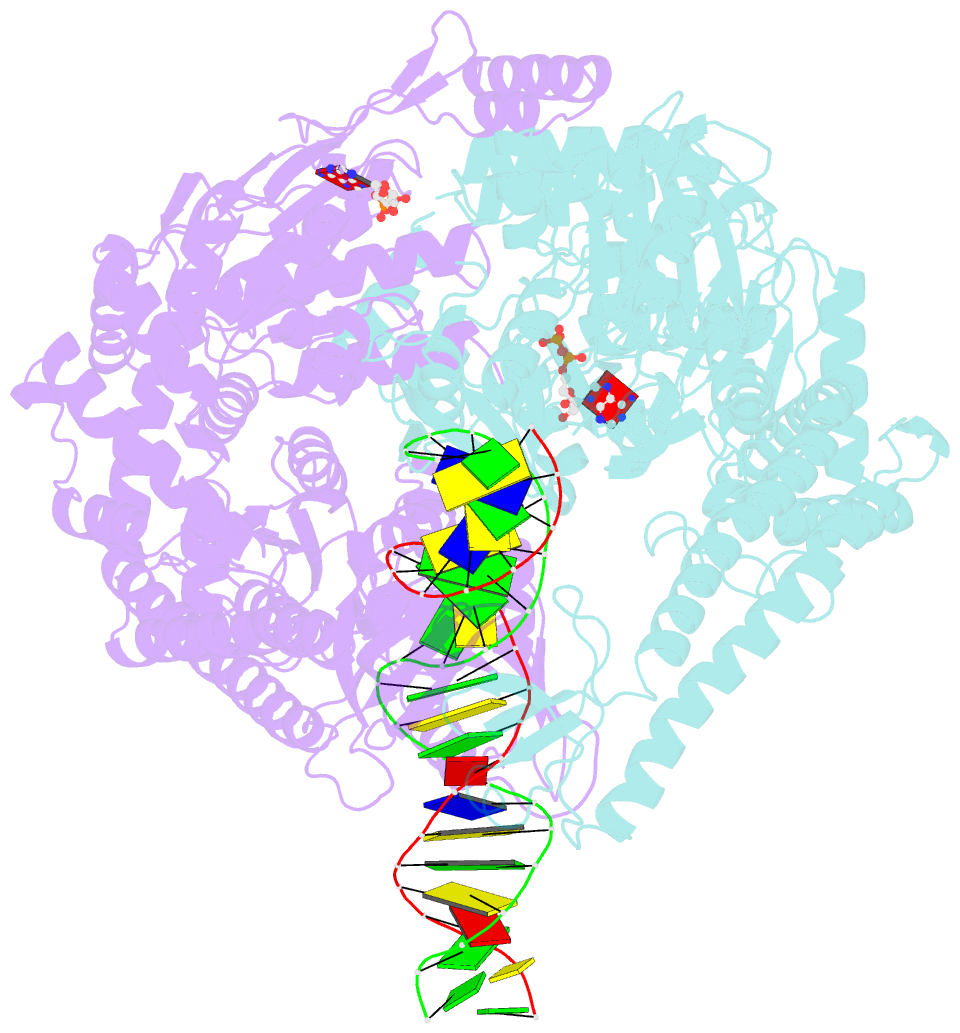
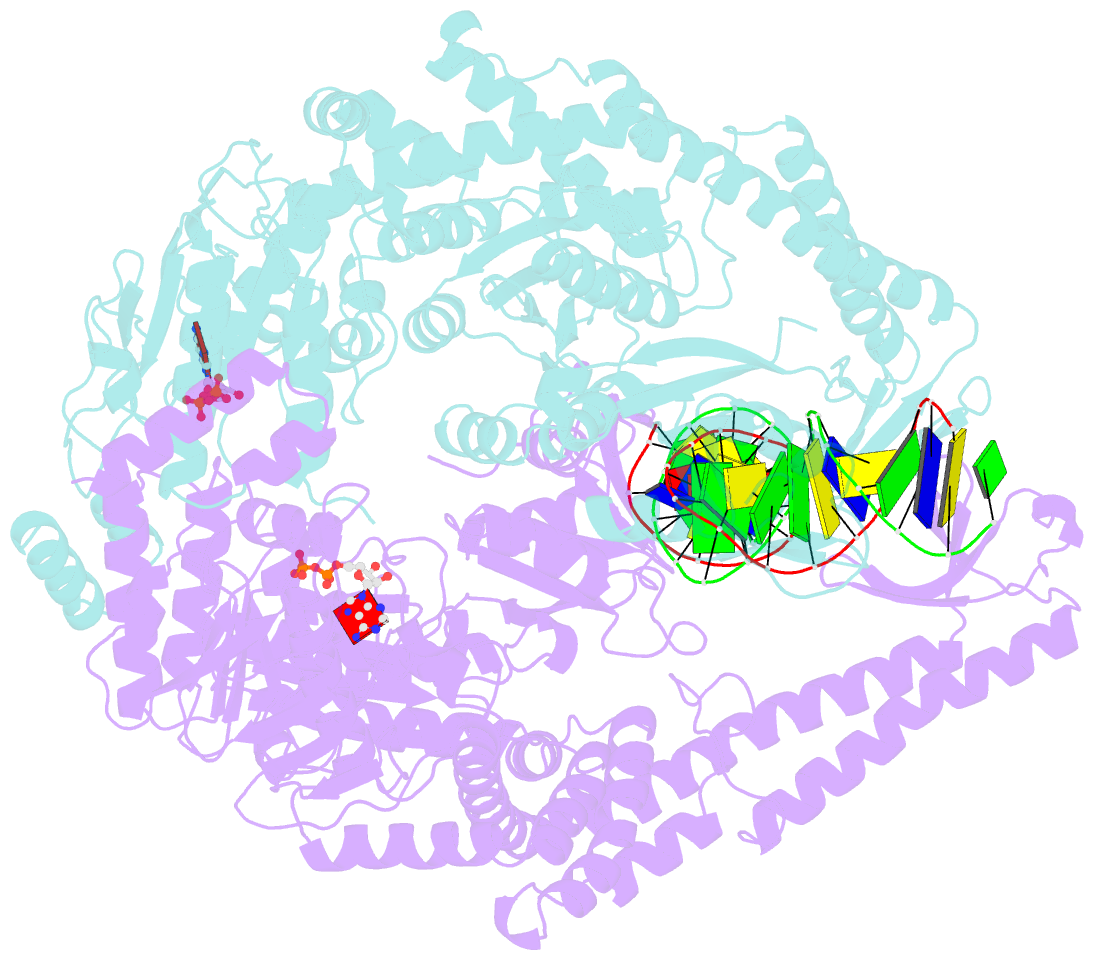
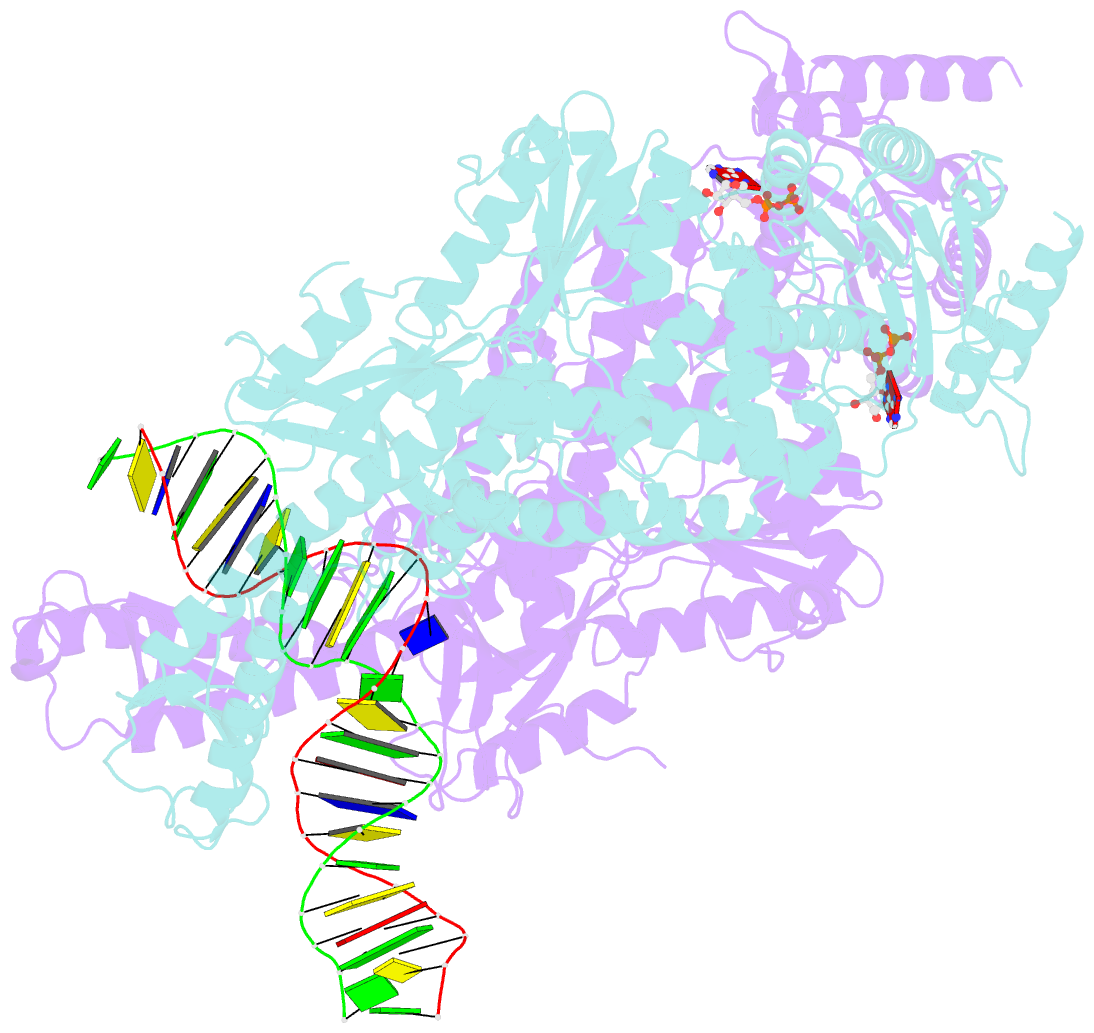
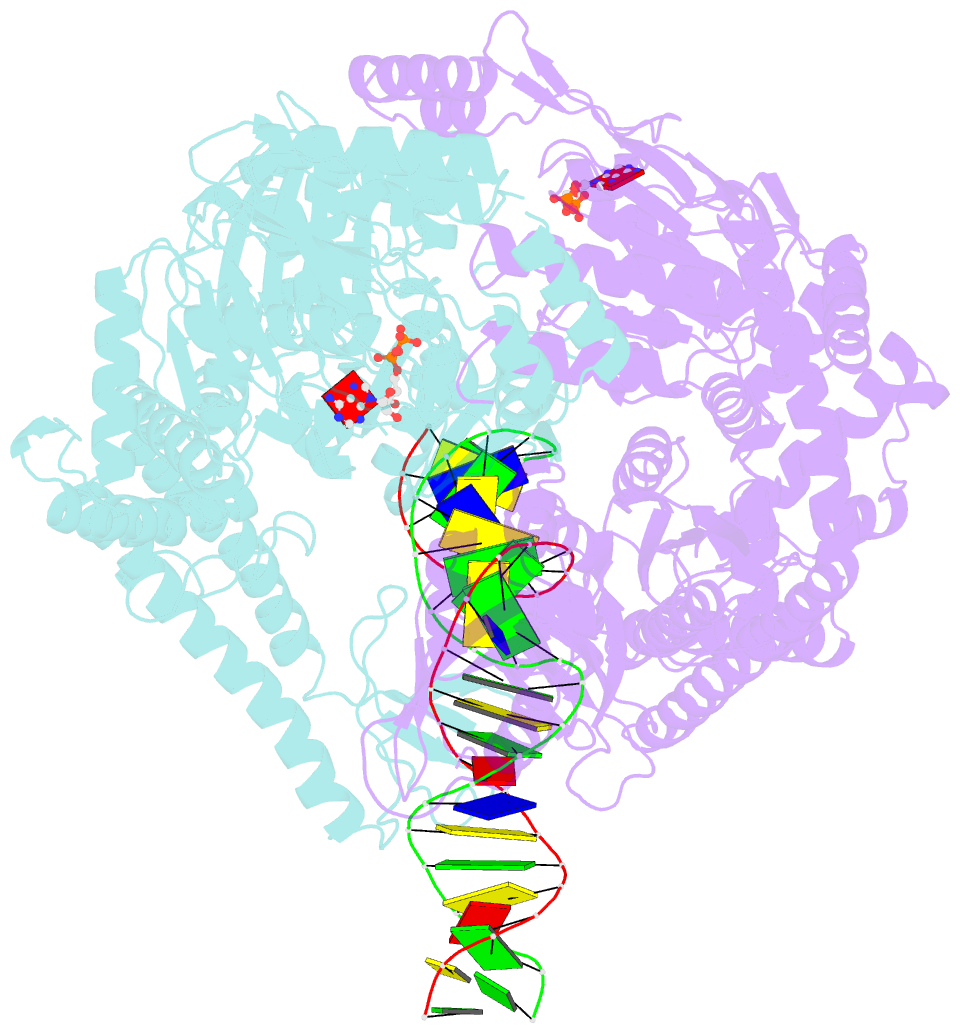
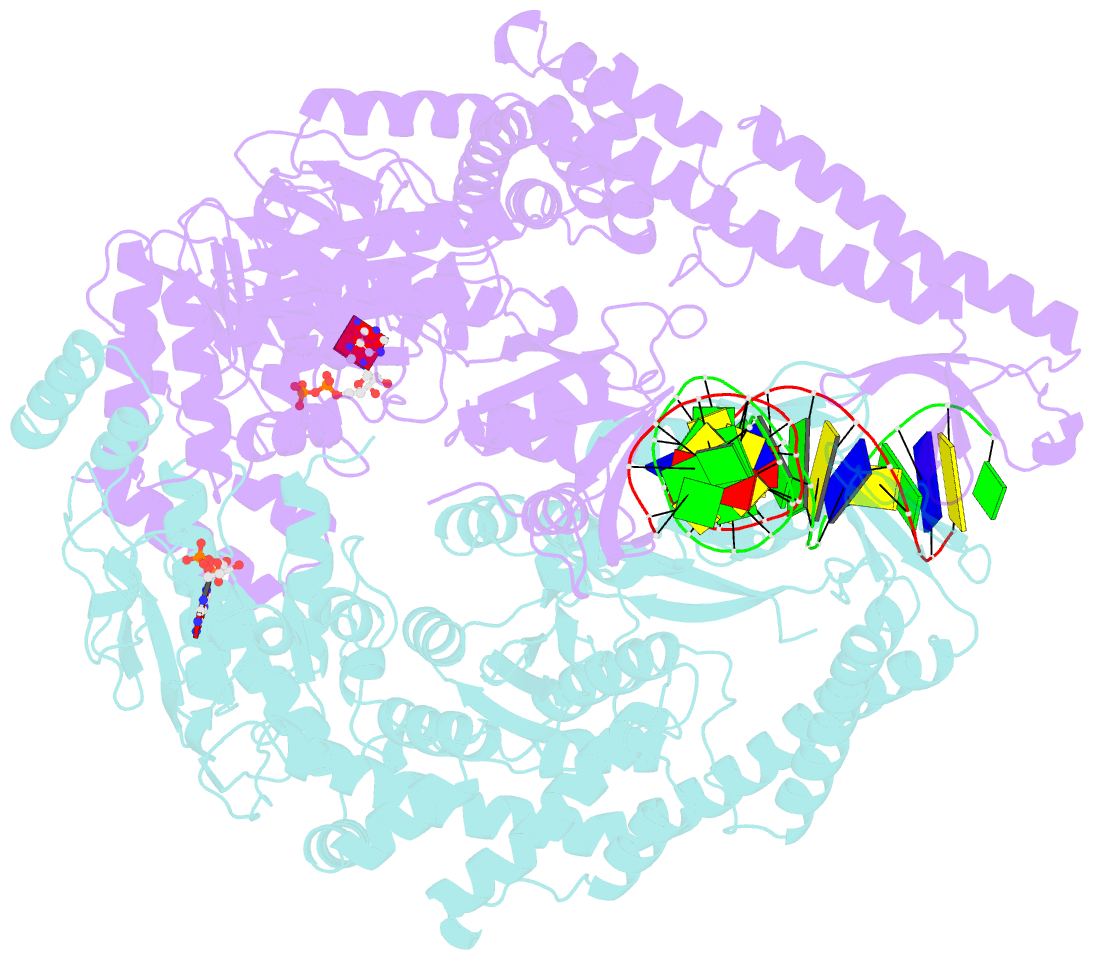
- Crystal structure of a taq muts-DNA-adp ternary complex
- Reference
- Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W (2001): "Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair." Mol.Cell, 7, 1-12. doi: 10.1016/S1097-2765(01)00149-6.
- Abstract
- The MutS protein initiates DNA mismatch repair by recognizing mispaired and unpaired bases embedded in duplex DNA and activating endo- and exonucleases to remove the mismatch. Members of the MutS family also possess a conserved ATPase activity that belongs to the ATP binding cassette (ABC) superfamily. Here we report the crystal structure of a ternary complex of MutS-DNA-ADP and assays of initiation of mismatch repair in conjunction with perturbation of the composite ATPase active site by mutagenesis. These studies indicate that MutS has to bind both ATP and the mismatch DNA simultaneously in order to activate the other mismatch repair proteins. We propose that the MutS ATPase activity plays a proofreading role in DNA mismatch repair, verification of mismatch recognition, and authorization of repair.