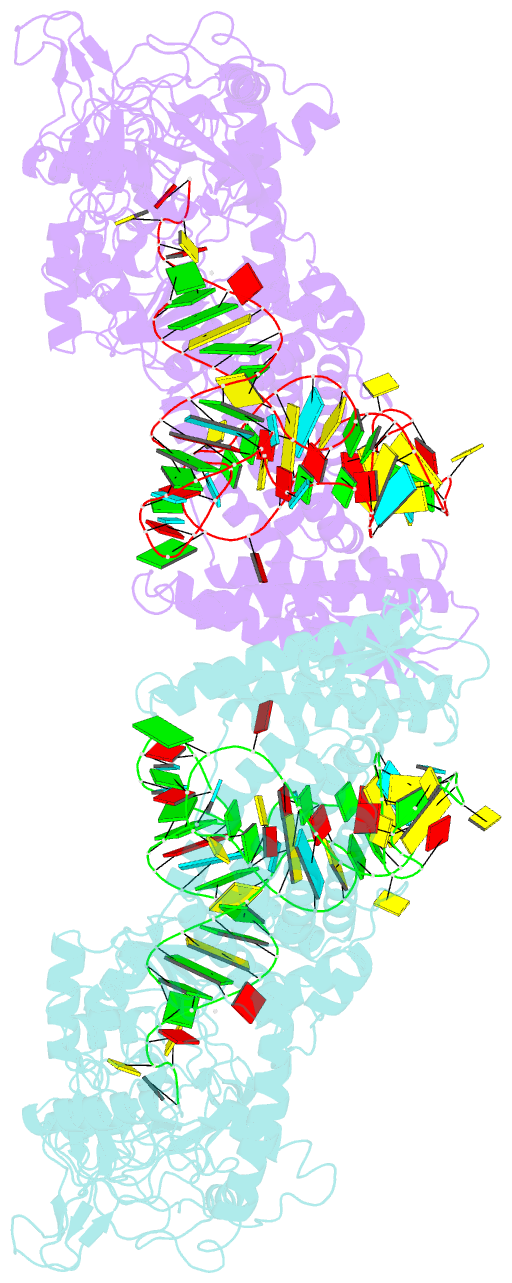
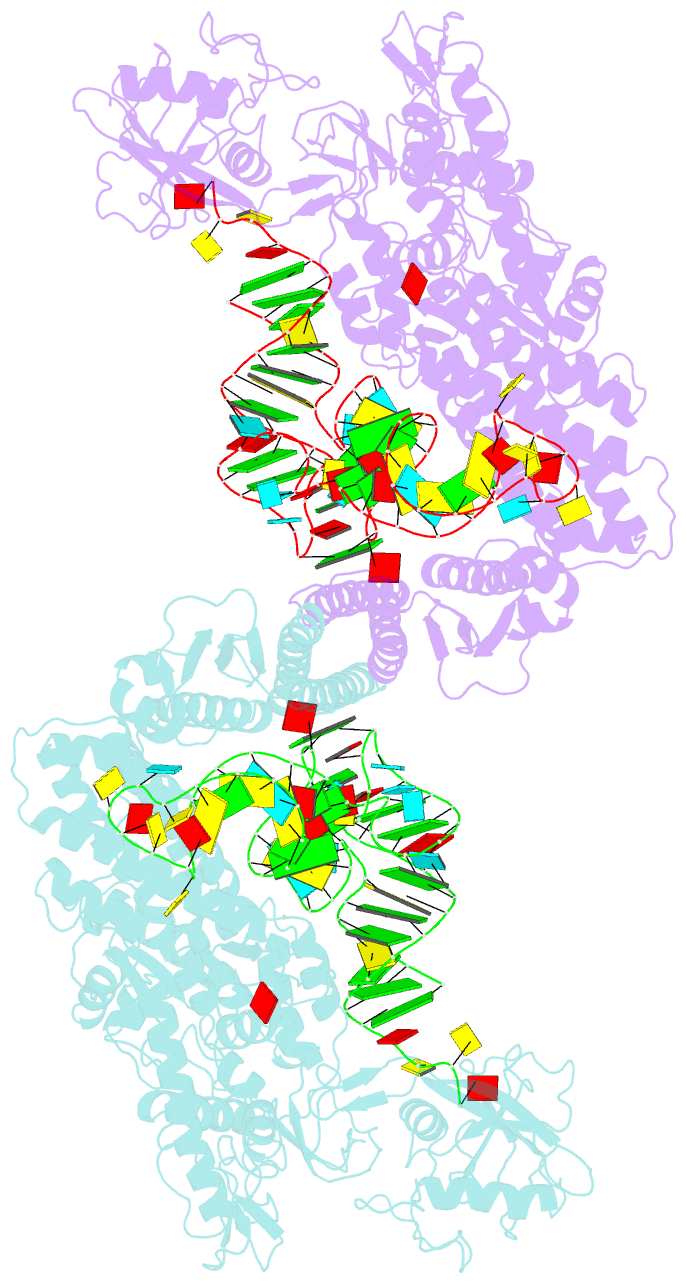
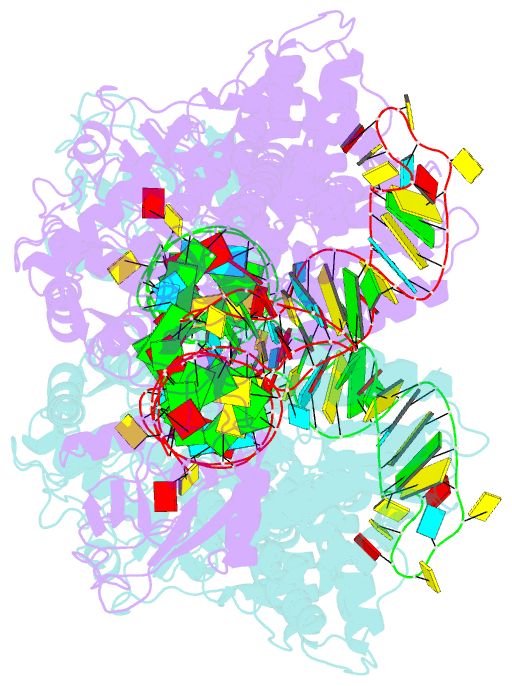
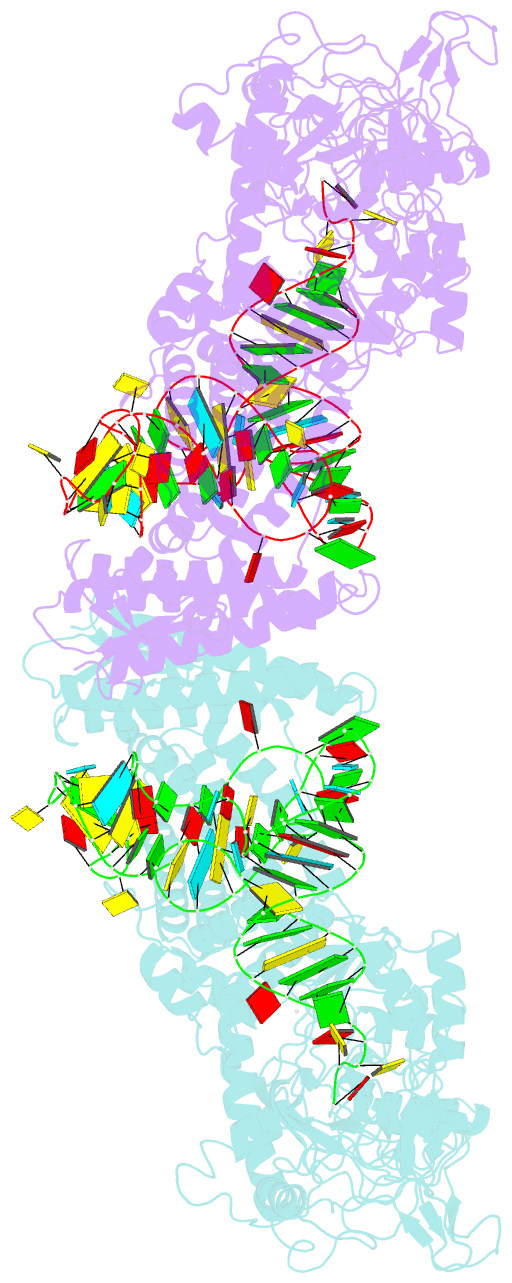
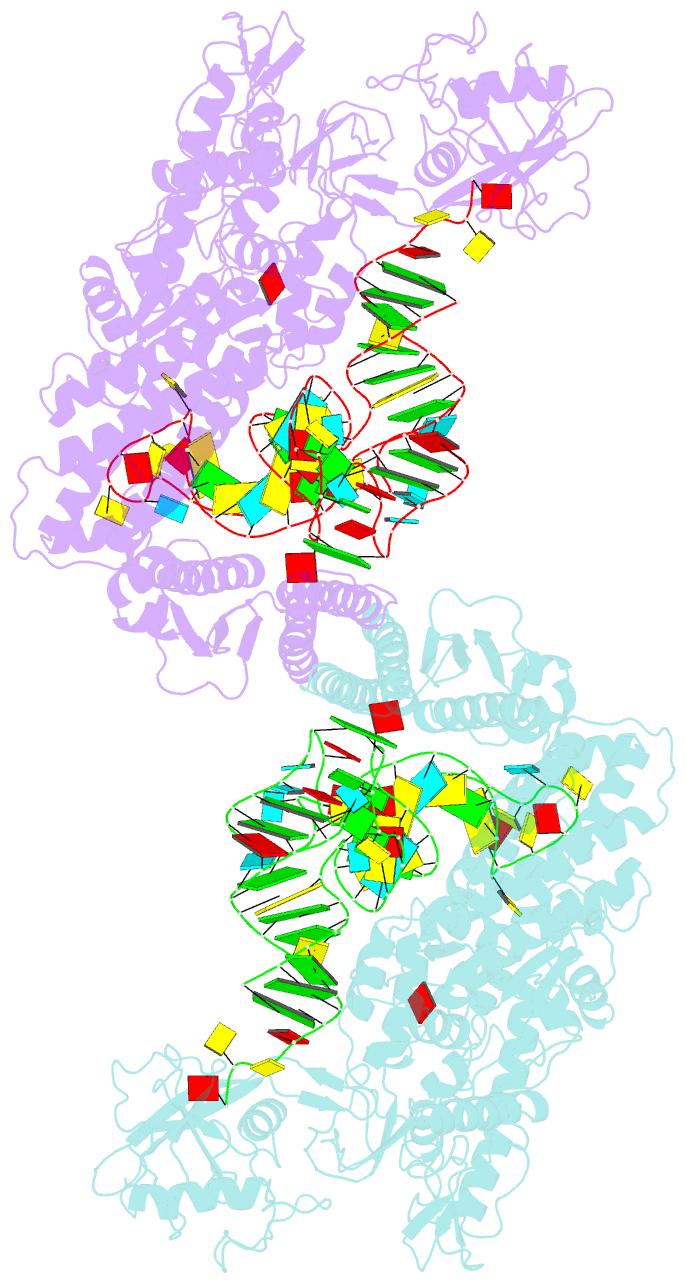
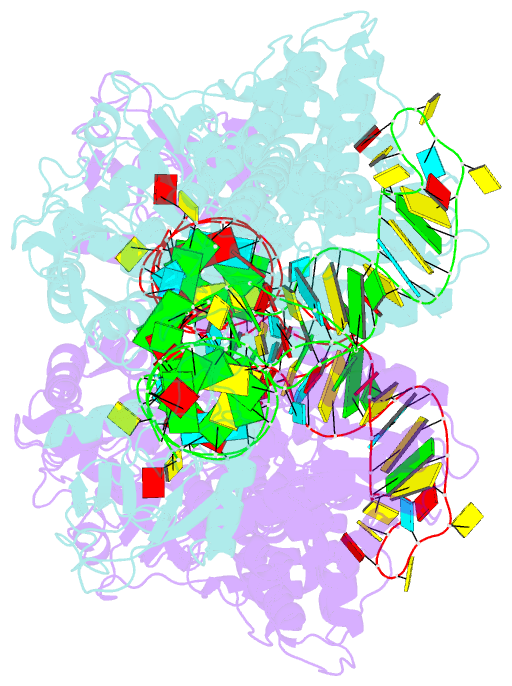
Summary information and primary citation
- PDB-id
- 1gax; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (2.9 Å)
- Summary
- Crystal structure of thermus thermophilus valyl-trna synthetase complexed with trna(val) and valyl-adenylate analogue
- Reference
- Fukai S, Nureki O, Sekine S, Shimada A, Tao J, Vassylyev DG, Yokoyama S (2000): "Structural basis for double-sieve discrimination of L-valine from L-isoleucine and L-threonine by the complex of tRNA(Val) and valyl-tRNA synthetase." Cell(Cambridge,Mass.), 103, 793-803. doi: 10.1016/S0092-8674(00)00182-3.
- Abstract
- Valyl-tRNA synthetase (ValRS) strictly discriminates the cognate L-valine from the larger L-isoleucine and the isosteric L-threonine by the tRNA-dependent "double sieve" mechanism. In this study, we determined the 2.9 A crystal structure of a complex of Thermus thermophilus ValRS, tRNA(Val), and an analog of the Val-adenylate intermediate. The analog is bound in a pocket, where Pro(41) allows accommodation of the Val and Thr moieties but precludes the Ile moiety (the first sieve), on the aminoacylation domain. The editing domain, which hydrolyzes incorrectly synthesized Thr-tRNA(Val), is bound to the 3' adenosine of tRNA(Val). A contiguous pocket was found to accommodate the Thr moiety, but not the Val moiety (the second sieve). Furthermore, another Thr binding pocket for Thr-adenylate hydrolysis was suggested on the editing domain.