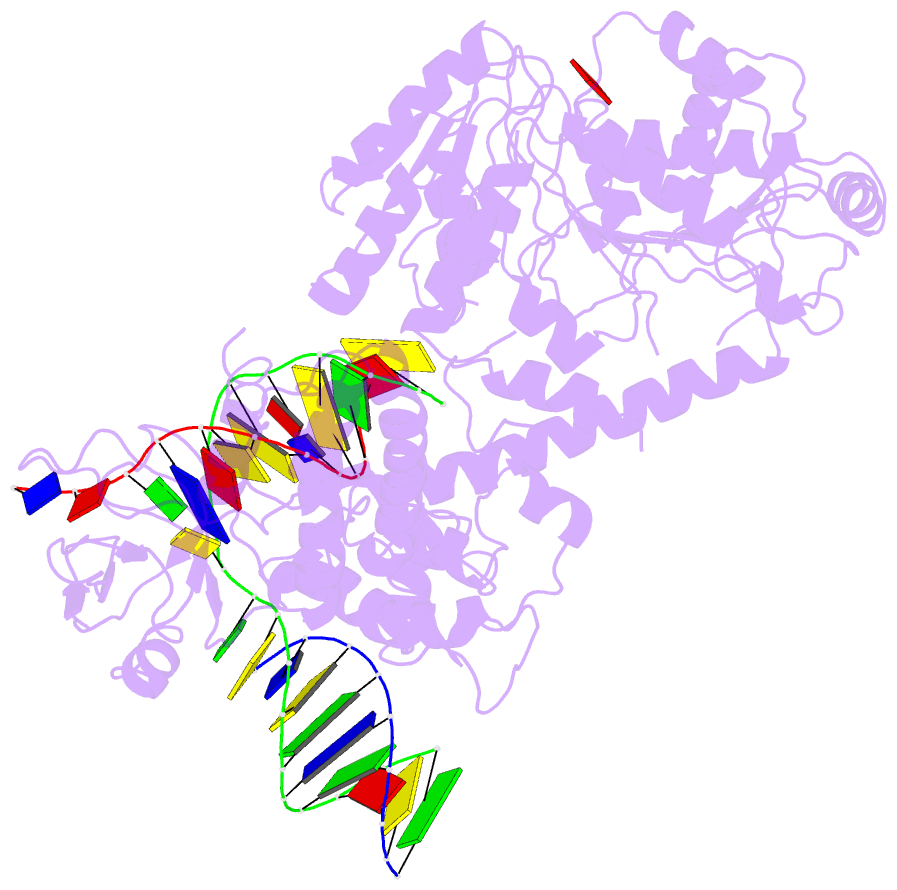
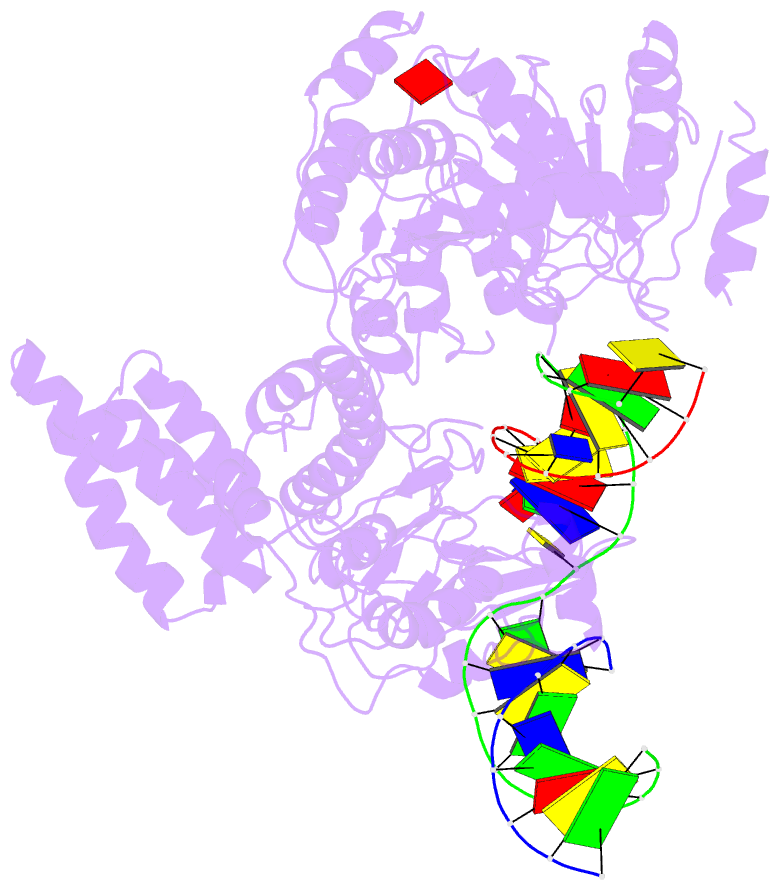
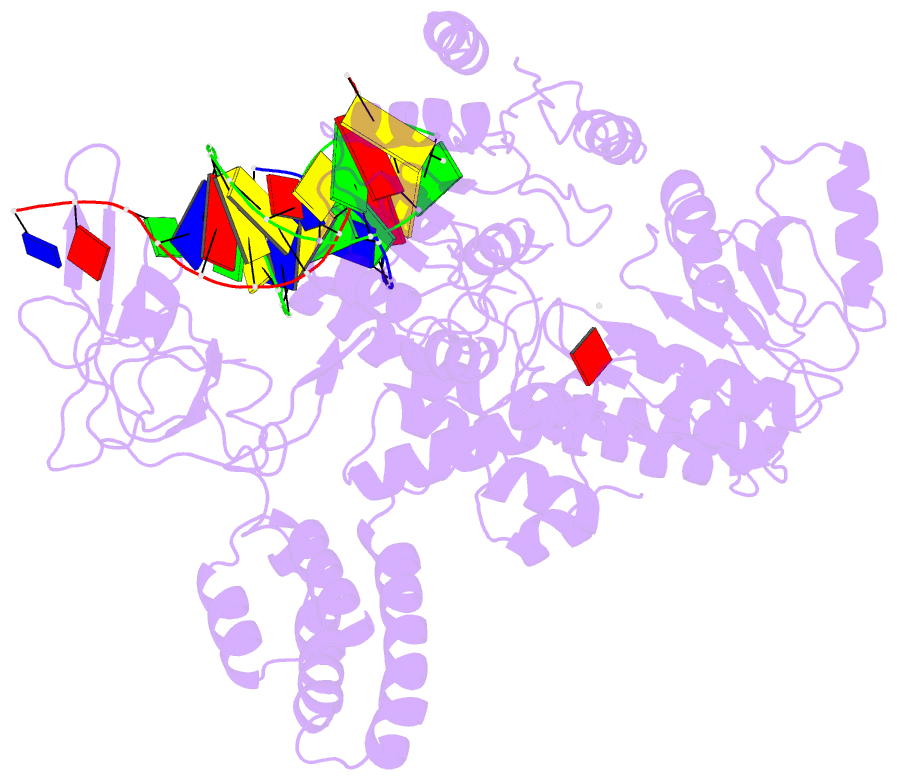
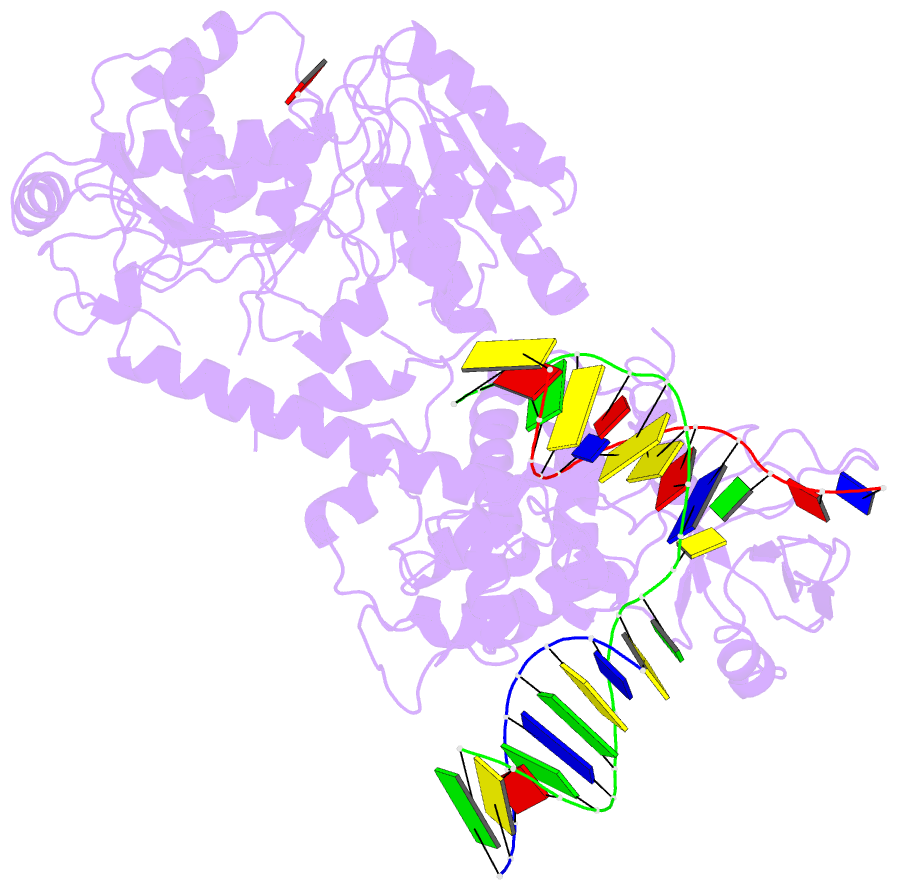
Summary information and primary citation
- PDB-id
- 1gm5; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- helicase
- Method
- X-ray (3.24 Å)
- Summary
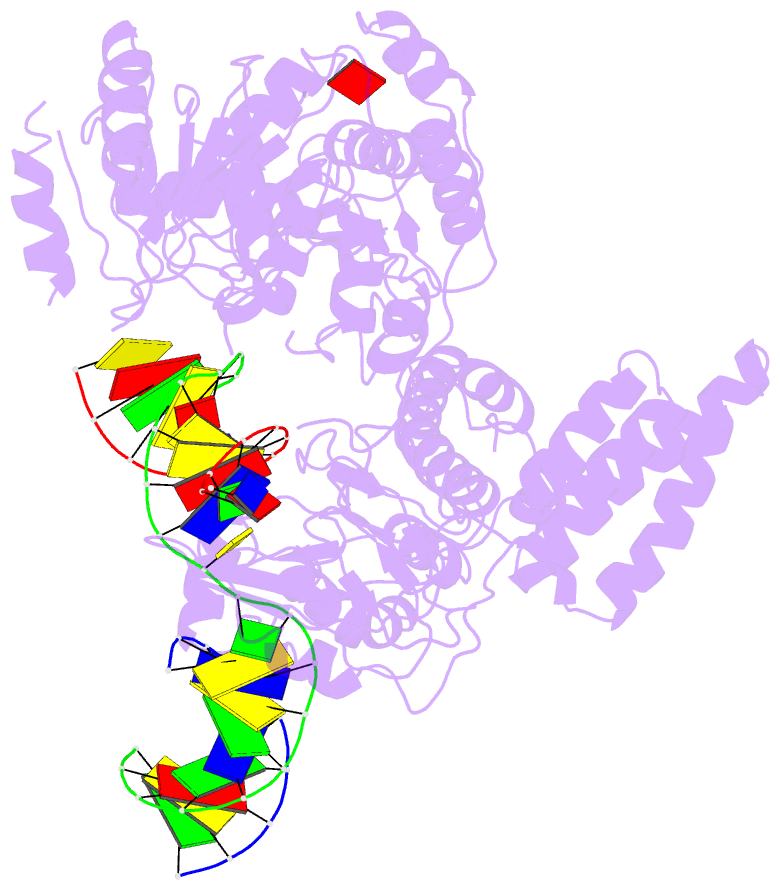
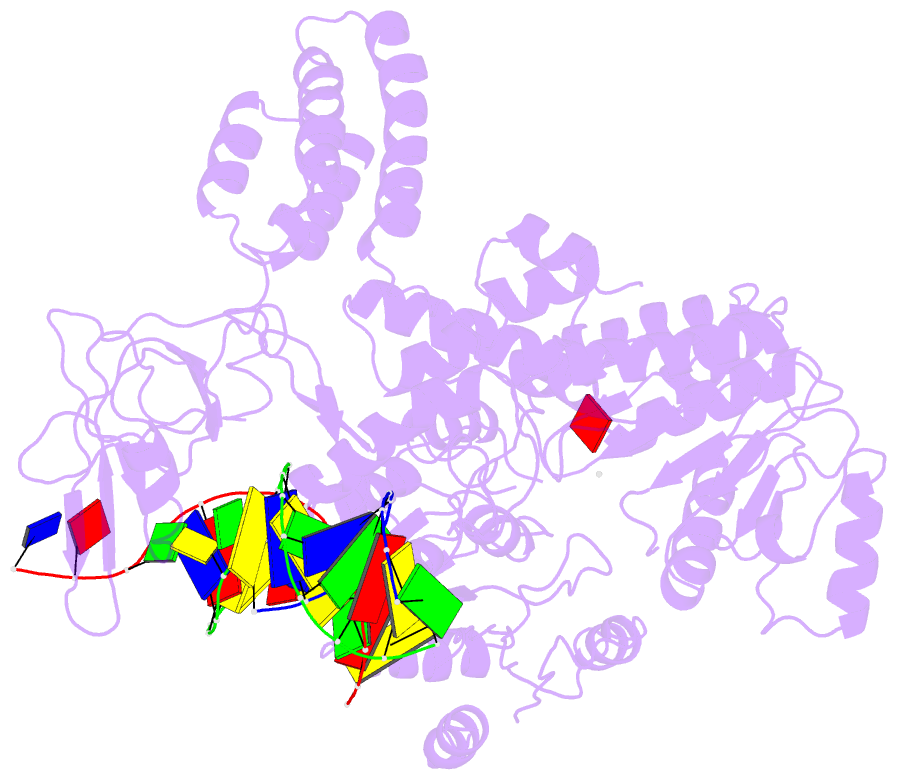
- Structure of recg bound to three-way DNA junction
- Reference
- Singleton MR, Scaife S, Wigley DB (2001): "Structural Analysis of DNA Replication Fork Reversal by Recg." Cell(Cambridge,Mass.), 107, 79. doi: 10.1016/S0092-8674(01)00501-3.
- Abstract
- The stalling of DNA replication forks that occurs as a consequence of encountering DNA damage is a critical problem for cells. RecG protein is involved in the processing of stalled replication forks, and acts by reversing the fork past the damage to create a four-way junction that allows template switching and lesion bypass. We have determined the crystal structure of RecG bound to a DNA substrate that mimics a stalled replication fork. The structure not only reveals the elegant mechanism used by the protein to recognize junctions but has also trapped the protein in the initial stage of fork reversal. We propose a mechanism for how forks are processed by RecG to facilitate replication fork restart. In addition, this structure suggests that the mechanism and function of the two largest helicase superfamilies are distinct.