Summary information and primary citation
- PDB-id
- 1gtf; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.75 Å)
- Summary
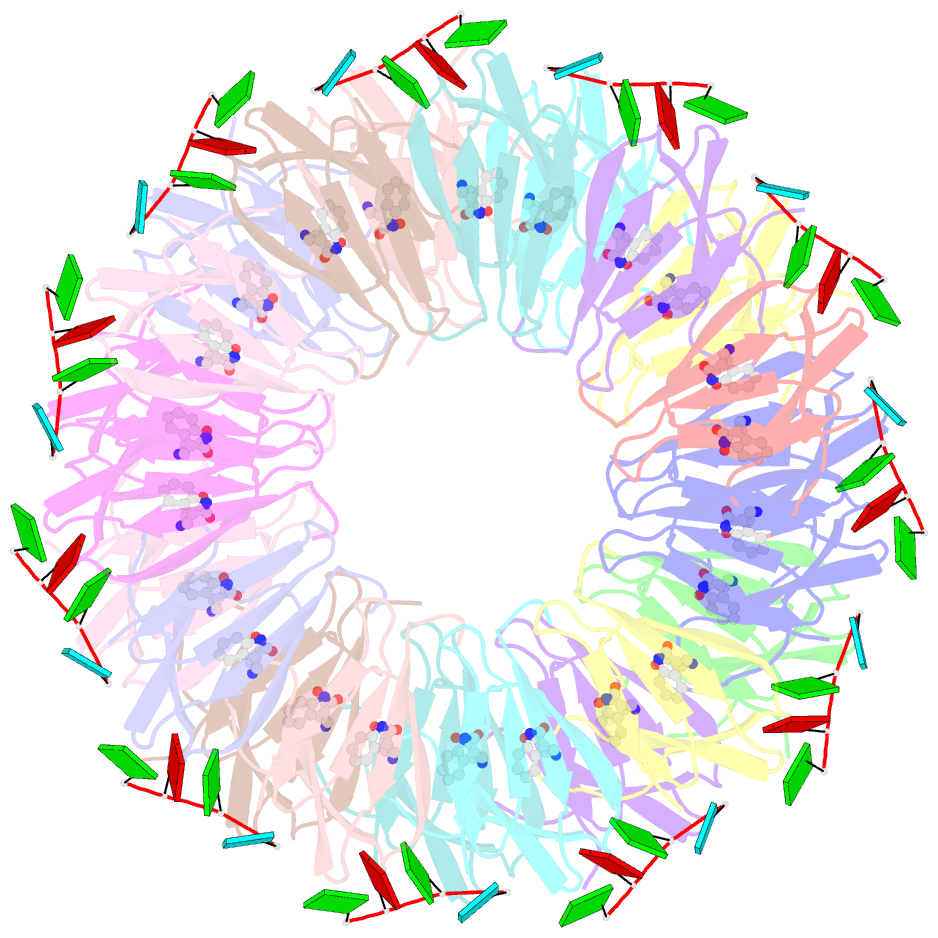
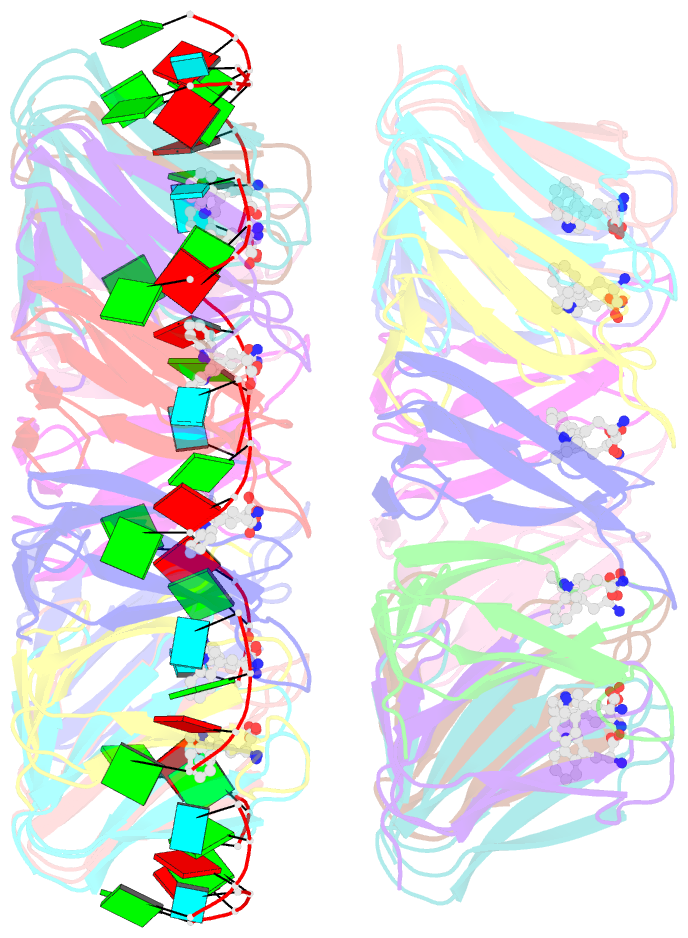
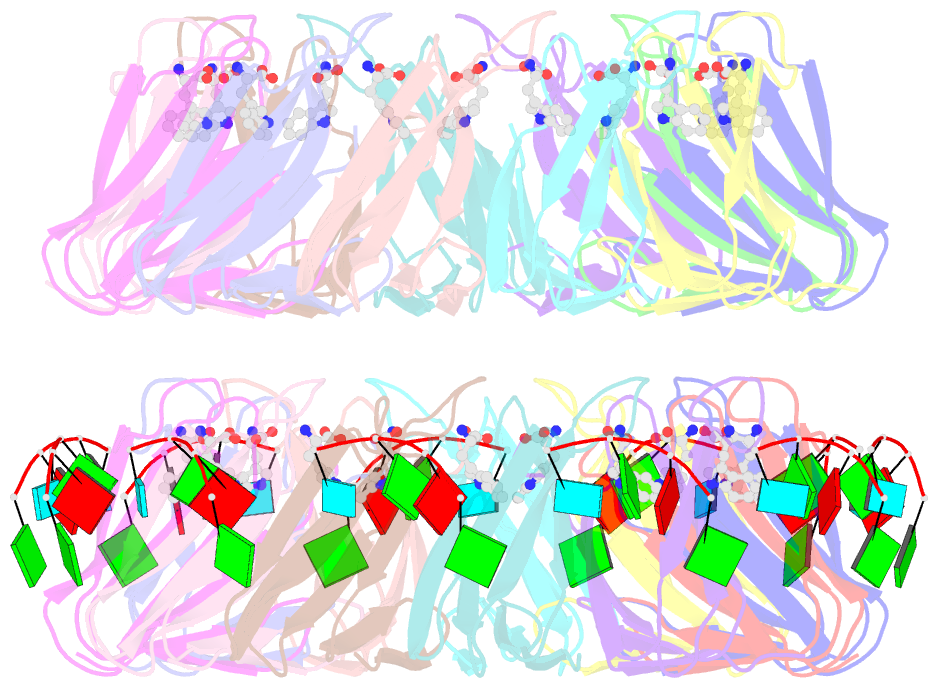
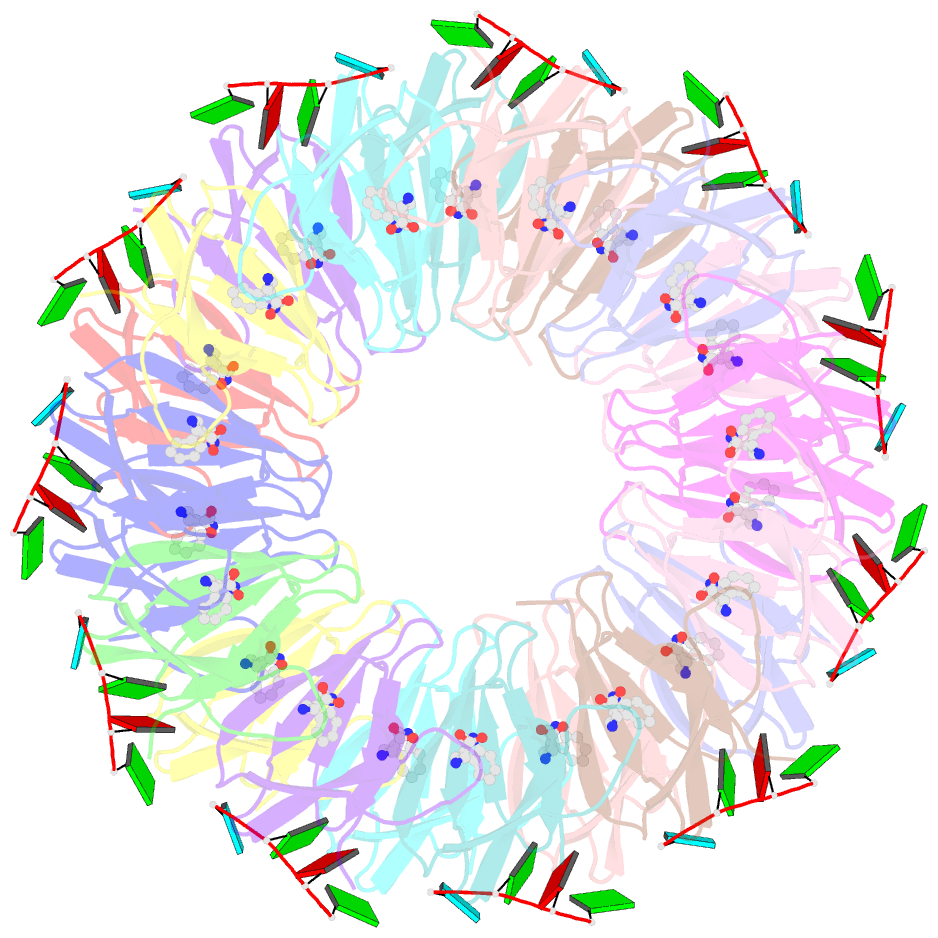
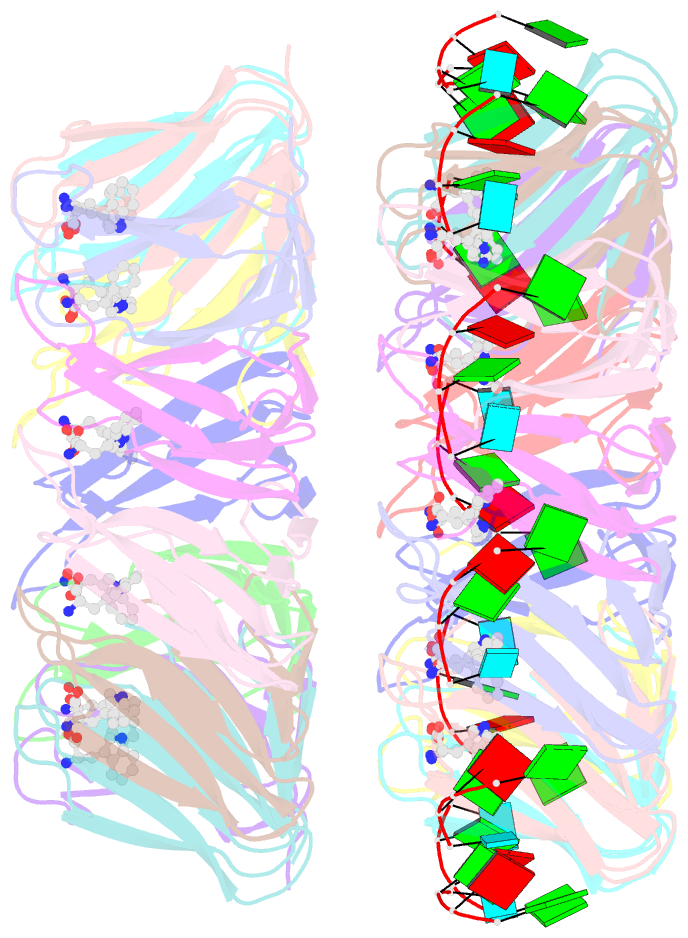
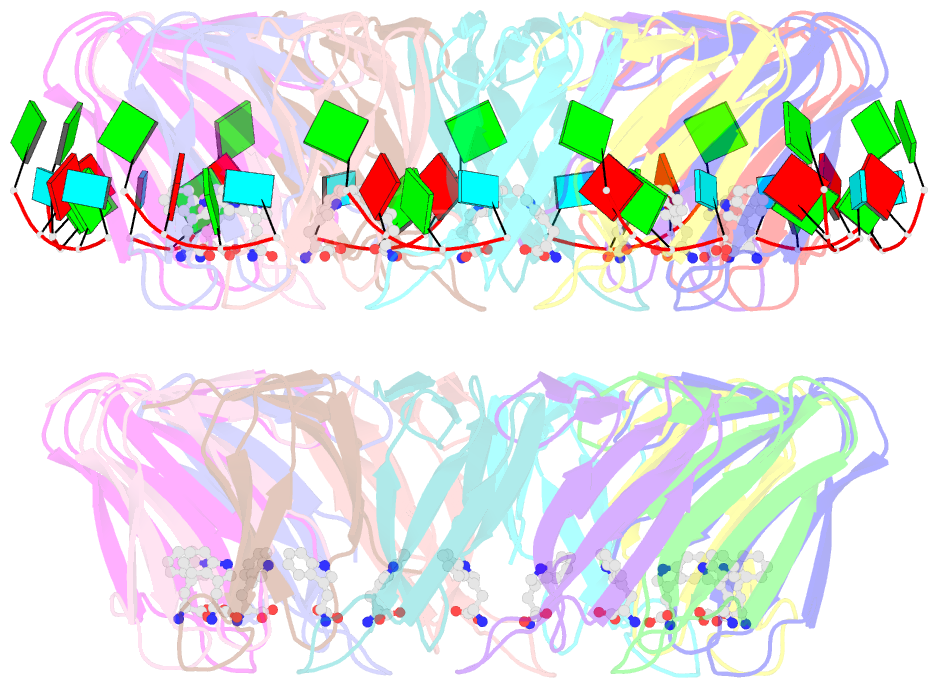
- The structure of the trp RNA-binding attenuation protein (trap) bound to a 53-nucleotide RNA molecule containing gaguu repeats
- Reference
- Hopcroft NH, Wendt AL, Gollnick P, Antson AA (2002): "Specificity of Trap-RNA Interactions: Crystal Structures of Two Complexes with Different RNA Sequences." Acta Crystallogr.,Sect.D, 58, 615. doi: 10.1107/S0907444902003189.
- Abstract
- The trp RNA-binding attenuation protein (TRAP) regulates expression of the tryptophan biosynthetic genes in bacilli by binding to the leader region of the nascent trp operon mRNA. When activated by binding tryptophan, the 11-subunit circular TRAP molecule binds to a target sequence consisting of 11 (G/U)AG repeats, separated by two or three variable 'spacer' nucleotides. Reported here are two crystal structures of TRAP bound to RNAs containing 11 GAG repeats separated by UU and CC spacer nucleotides, determined at 1.75 and 2.50 A resolution, respectively. These show the spacer regions of the RNA molecules to be highly flexible, making no direct hydrogen-bonding contacts with the protein. Comparison of these structures with the previous structure of TRAP bound to (GAGAU)(10)GAG RNA, in which the spacer nucleotides stack with each other close to the protein surface, shows that the RNA can adopt different conformations depending on the sequence of the spacer regions. This gives insight into the structural basis of the specificity of TRAP and into the mechanism of binding.