Summary information and primary citation
- PDB-id
-
1h89;
DSSR-derived features in text and
JSON formats; DNAproDB
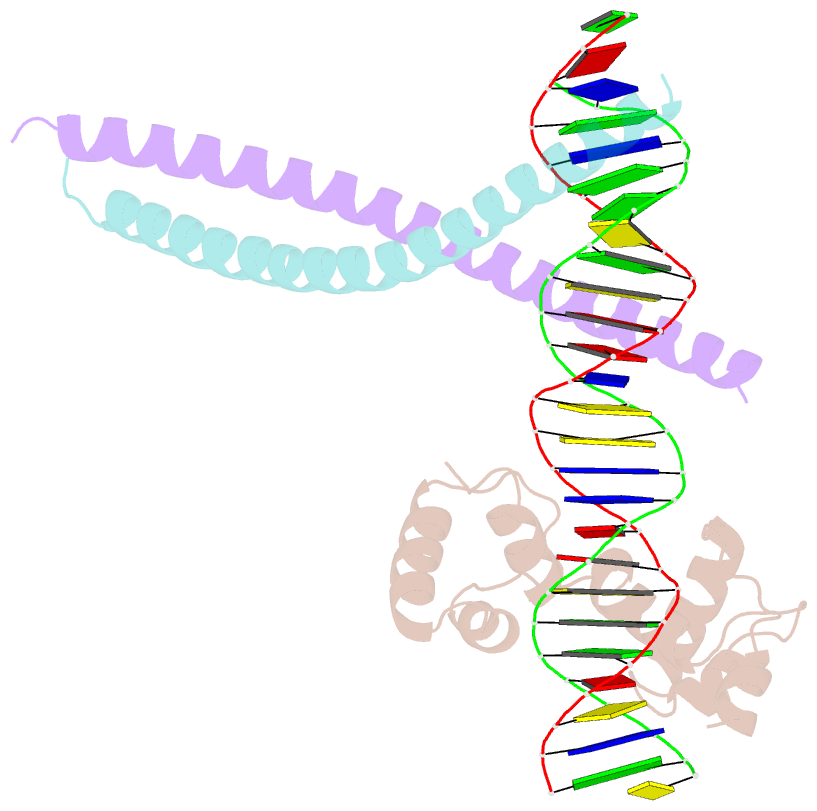
- Class
- transcription-DNA
- Method
- X-ray (2.45 Å)
- Summary
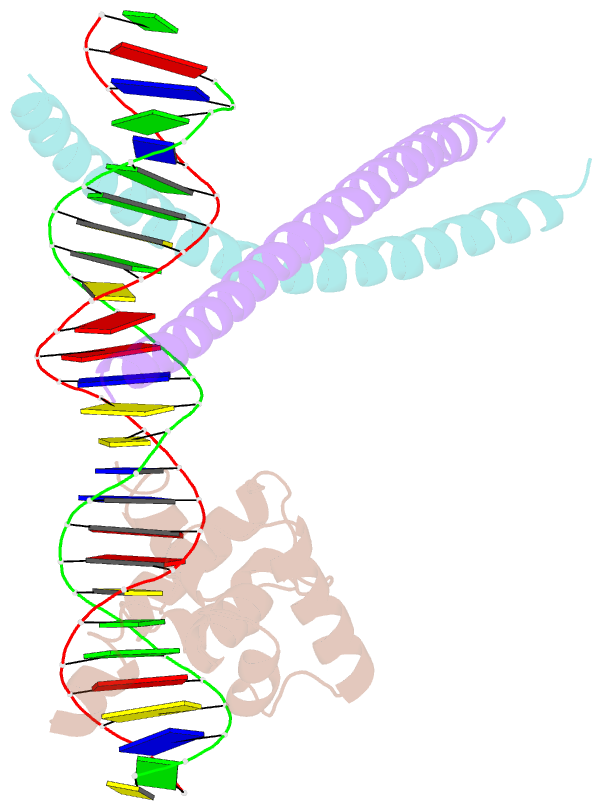
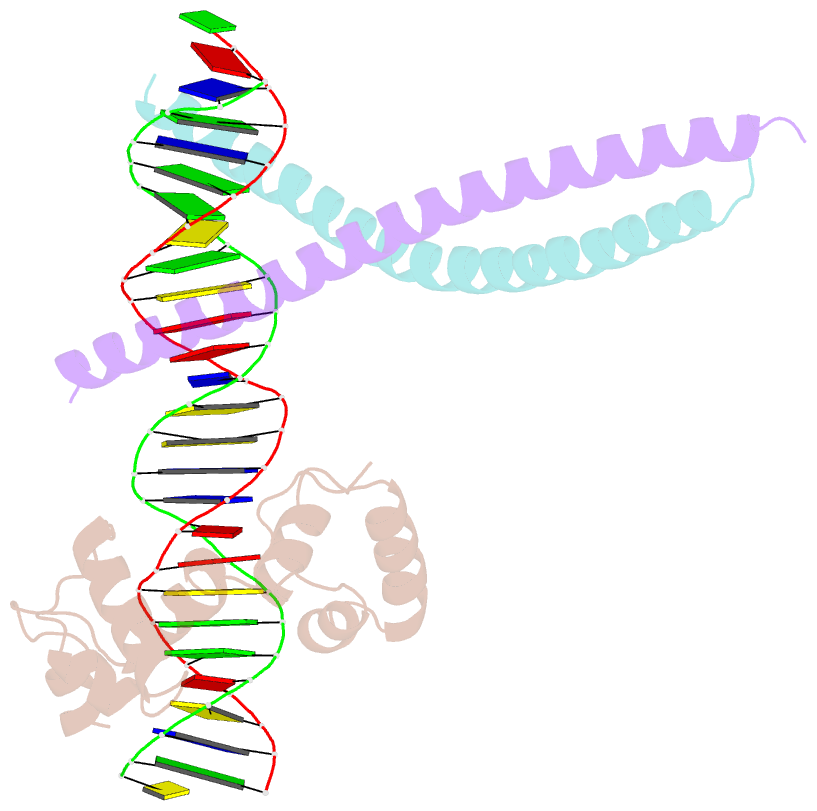
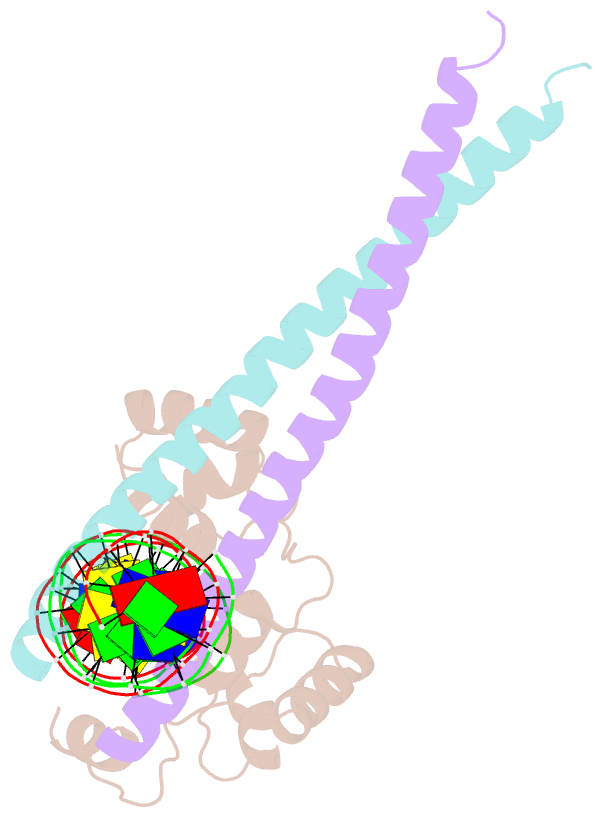
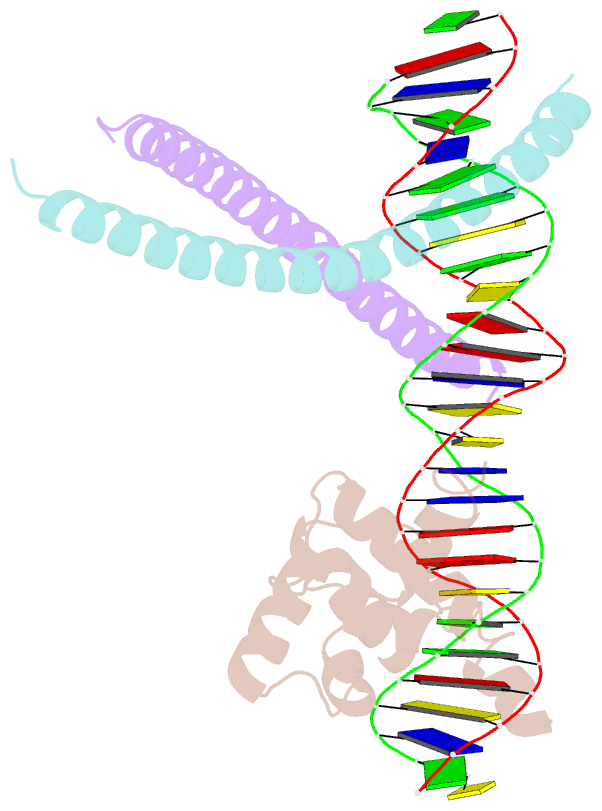
- Crystal structure of ternary protein-DNA complex2
- Reference
-
Tahirov TH, Sato K, Ichikawa-Iwata E, Sasaki M,
Inoue-Bungo T, Shiina M, Kimura K, Takata S, Fujikawa A,
Morii H, Kumasaka T, Yamamoto M, Ishii S, Ogata K (2002):
"Mechanism
of C-Myb-C/Ebpbeta Cooperation from Separated Sites on a
Promoter." Cell(Cambridge,Mass.),
108, 57. doi: 10.1016/S0092-8674(01)00636-5.
- Abstract
- c-Myb, but not avian myeloblastosis virus (AMV) v-Myb,
cooperates with C/EBP beta to regulate transcription of
myeloid-specific genes. To assess the structural basis for
that difference, we determined the crystal structures of
complexes comprised of the c-Myb or AMV v-Myb DNA-binding
domain (DBD), the C/EBP beta DBD, and a promoter DNA
fragment. Within the c-Myb complex, a DNA-bound C/EBP beta
interacts with R2 of c-Myb bound to a different DNA
fragment; point mutations in v-Myb R2 eliminate such
interaction within the v-Myb complex. GST pull-down assays,
luciferase trans-activation assays, and atomic force
microscopy confirmed that the interaction of c-Myb and
C/EBP beta observed in crystal mimics their long range
interaction on the promoter, which is accompanied by
intervening DNA looping.