Summary information and primary citation
- PDB-id
- 1hlv; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.5 Å)
- Summary
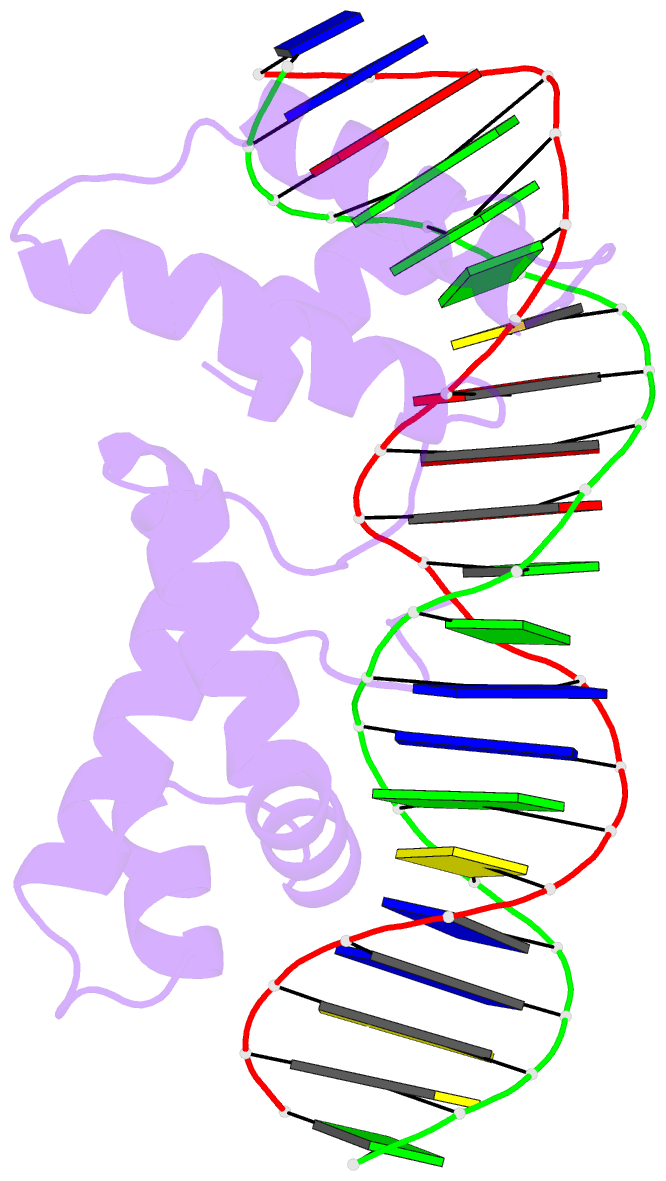
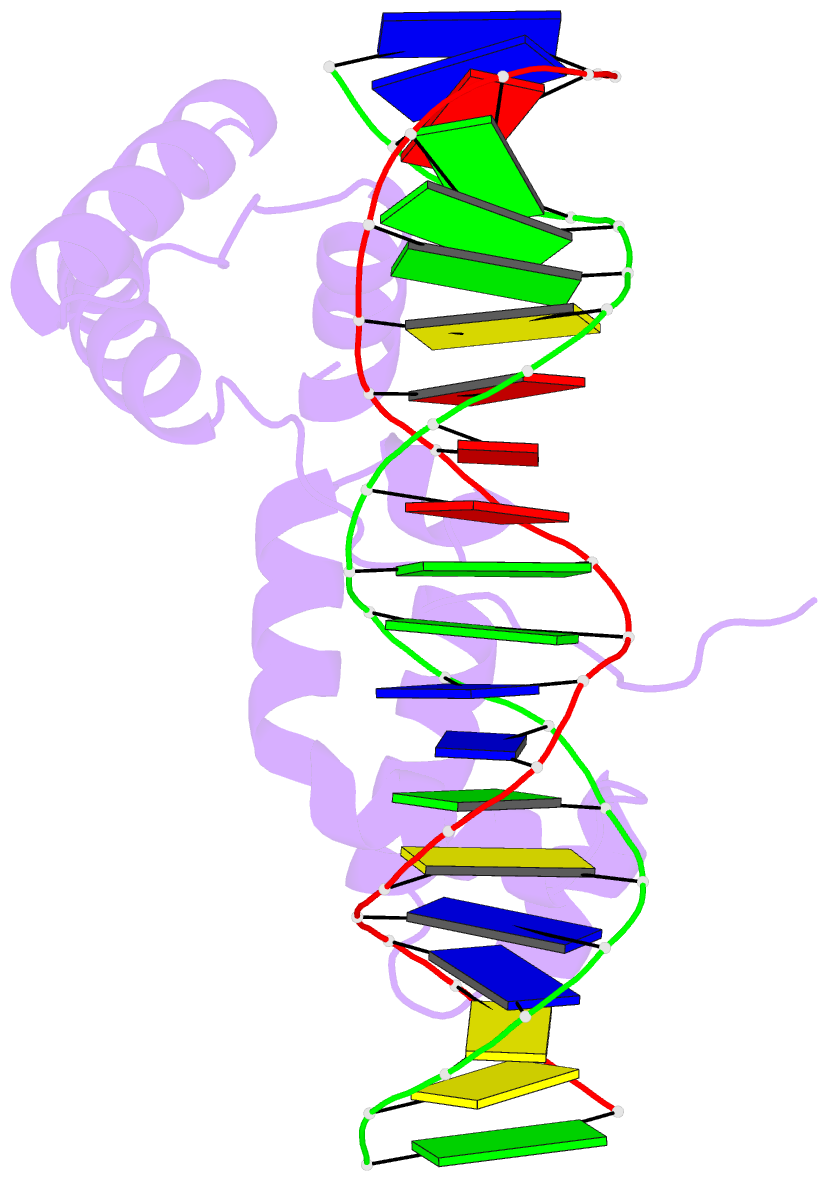
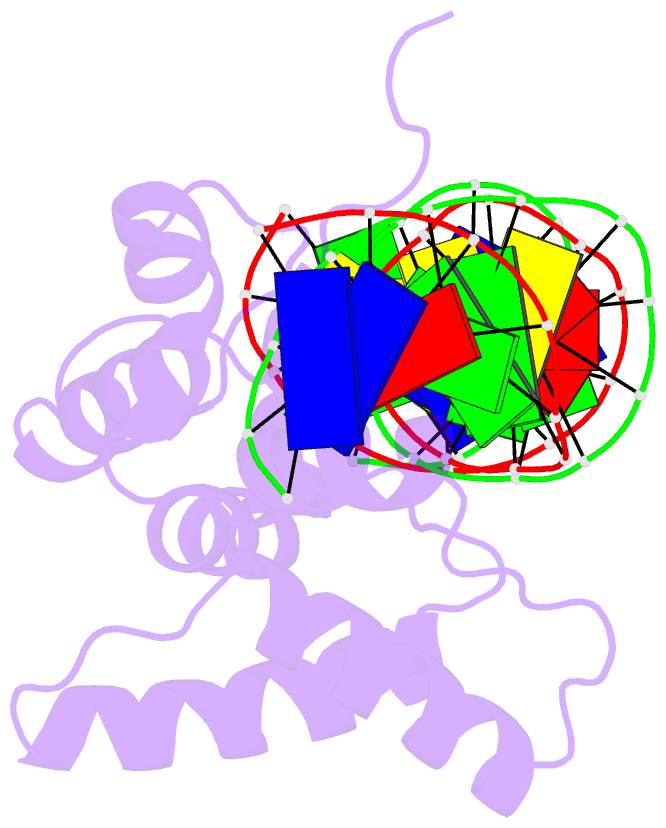
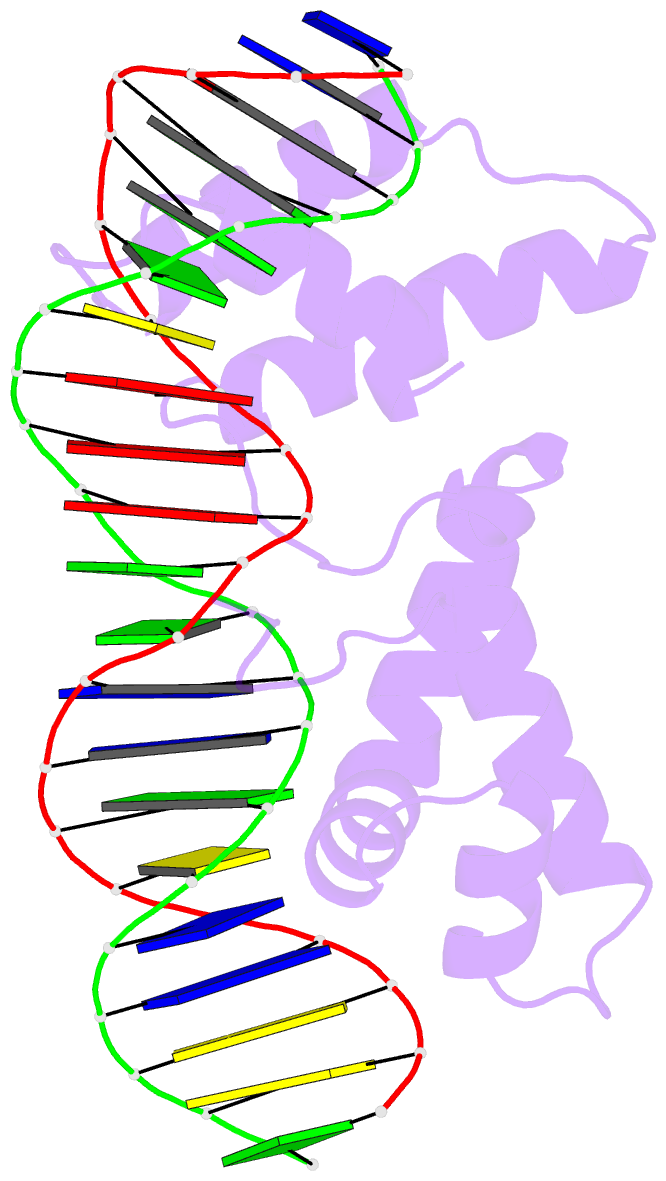
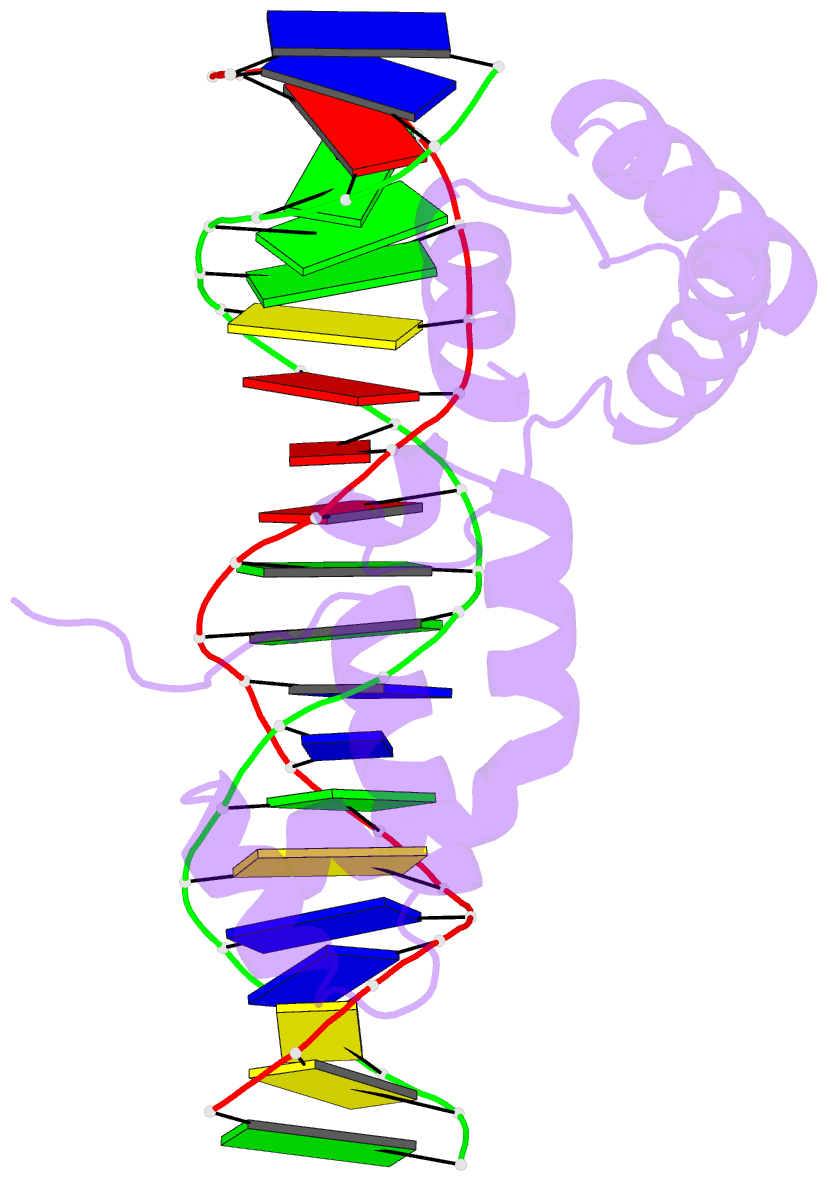
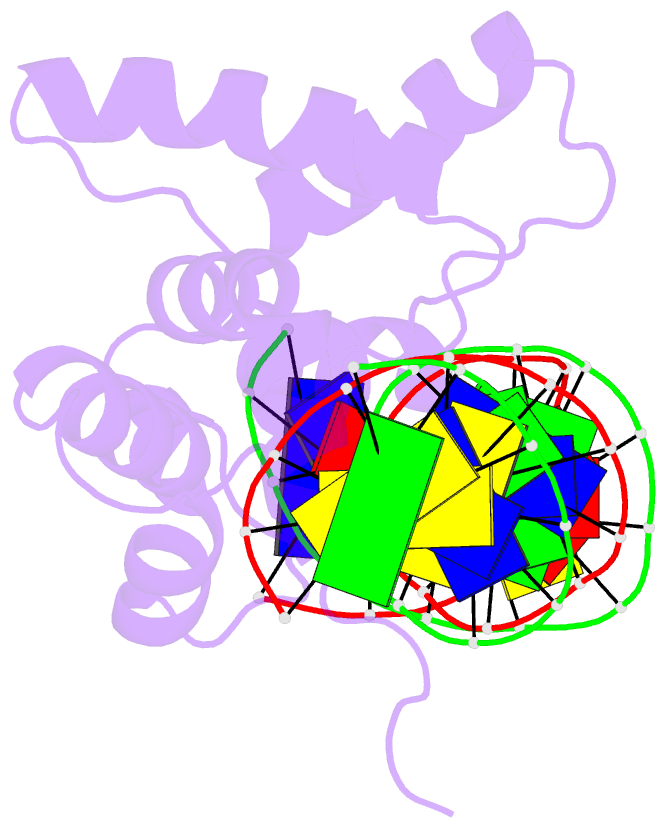
- Crystal structure of cenp-b(1-129) complexed with the cenp-b box DNA
- Reference
- Tanaka Y, Nureki O, Kurumizaka H, Fukai S, Kawaguchi S, Ikuta M, Iwahara J, Okazaki T, Yokoyama S (2001): "Crystal structure of the CENP-B protein-DNA complex: the DNA-binding domains of CENP-B induce kinks in the CENP-B box DNA." EMBO J., 20, 6612-6618. doi: 10.1093/emboj/20.23.6612.
- Abstract
- The human centromere protein B (CENP-B), one of the centromere components, specifically binds a 17 bp sequence (the CENP-B box), which appears in every other alpha-satellite repeat. In the present study, the crystal structure of the complex of the DNA-binding region (129 residues) of CENP-B and the CENP-B box DNA has been determined at 2.5 A resolution. The DNA-binding region forms two helix-turn-helix domains, which are bound to adjacent major grooves of the DNA. The DNA is kinked at the two recognition helix contact sites, and the DNA region between the kinks is straight. Among the major groove protein-bound DNAs, this 'kink-straight-kink' bend contrasts with ordinary 'round bends' (gradual bending between two protein contact sites). The larger kink (43 degrees ) is induced by a novel mechanism, 'phosphate bridging by an arginine-rich helix': the recognition helix with an arginine cluster is inserted perpendicularly into the major groove and bridges the groove through direct interactions with the phosphate groups. The overall bending angle is 59 degrees, which may be important for the centromere-specific chromatin structure.