Summary information and primary citation
- PDB-id
- 1hys; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA-RNA hybrid
- Method
- X-ray (3.0 Å)
- Summary
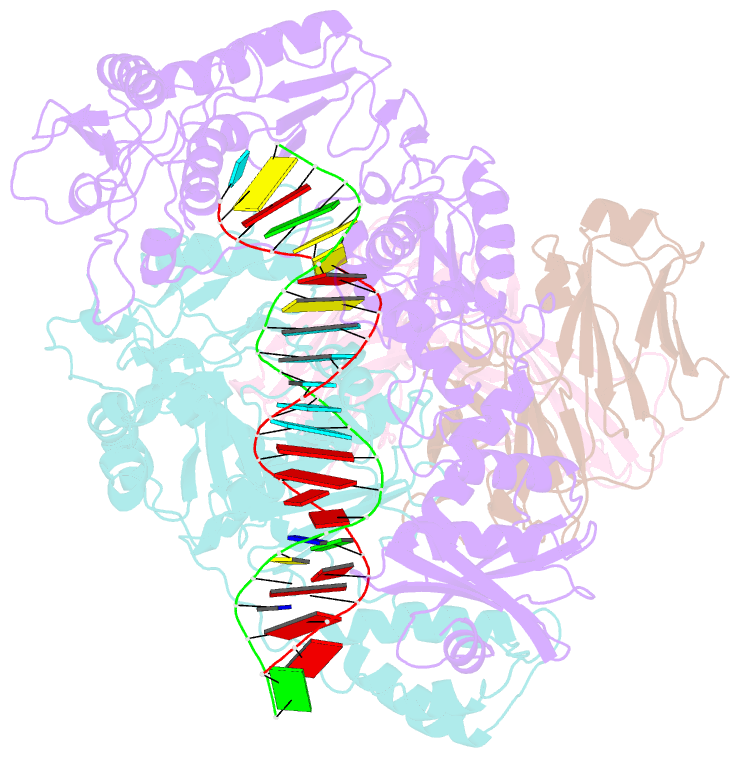
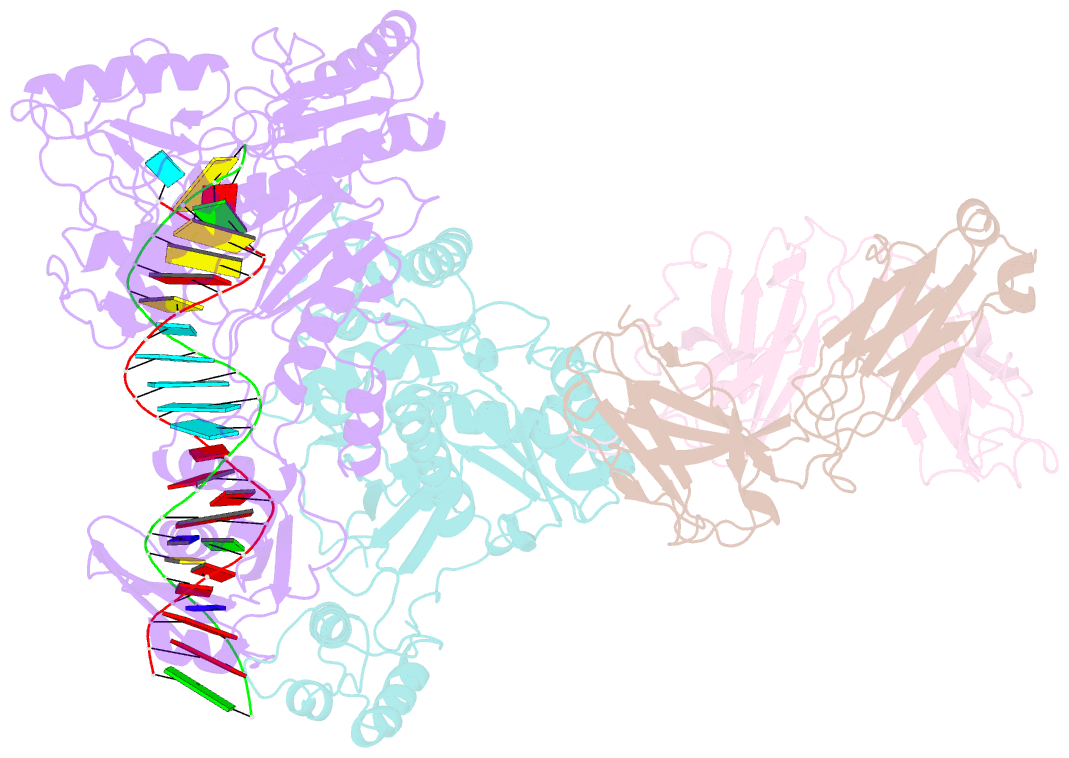
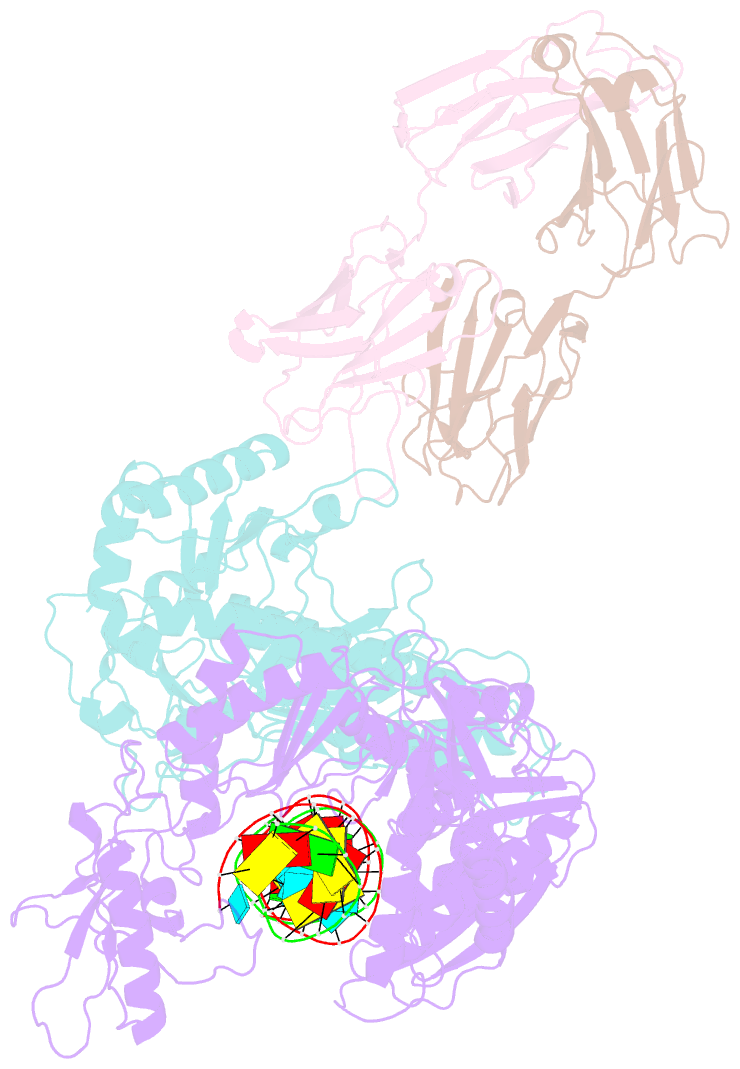
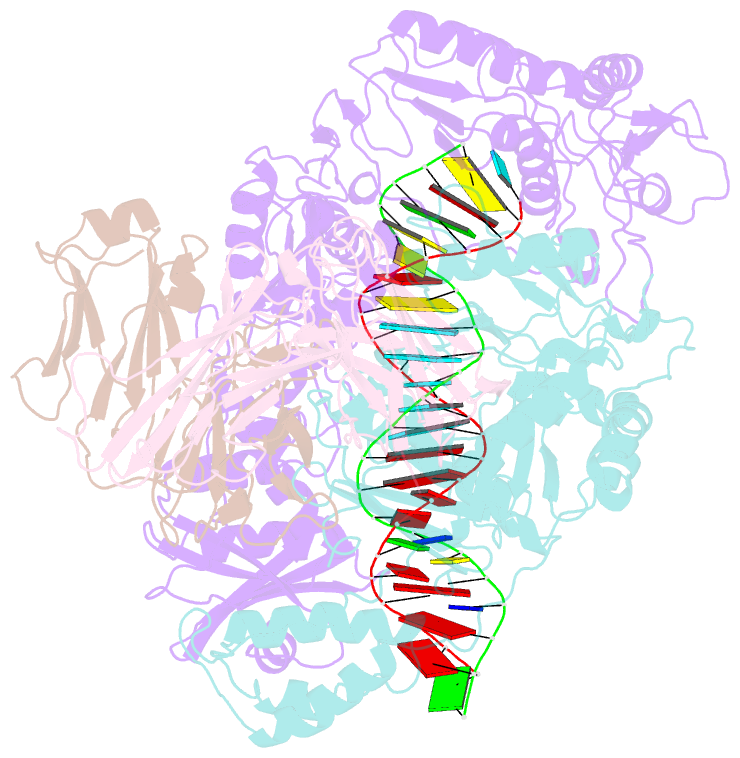
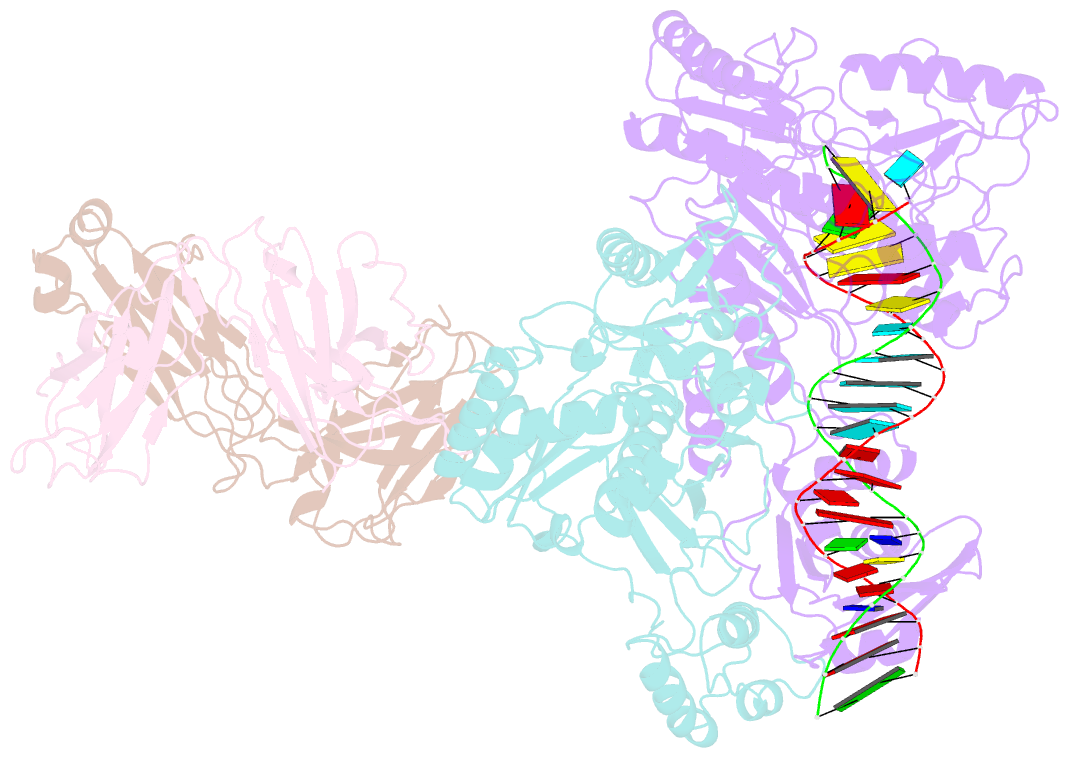
- Crystal structure of hiv-1 reverse transcriptase in complex with a polypurine tract RNA:DNA
- Reference
- Sarafianos SG, Das K, Tantillo C, Clark Jr AD, Ding J, Whitcomb JM, Boyer PL, Hughes SH, Arnold E (2001): "Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA." EMBO J., 20, 1449-1461. doi: 10.1093/emboj/20.6.1449.
- Abstract
- We have determined the 3.0 A resolution structure of wild-type HIV-1 reverse transcriptase in complex with an RNA:DNA oligonucleotide whose sequence includes a purine-rich segment from the HIV-1 genome called the polypurine tract (PPT). The PPT is resistant to ribonuclease H (RNase H) cleavage and is used as a primer for second DNA strand synthesis. The 'RNase H primer grip', consisting of amino acids that interact with the DNA primer strand, may contribute to RNase H catalysis and cleavage specificity. Cleavage specificity is also controlled by the width of the minor groove and the trajectory of the RNA:DNA, both of which are sequence dependent. An unusual 'unzipping' of 7 bp occurs in the adenine stretch of the PPT: an unpaired base on the template strand takes the base pairing out of register and then, following two offset base pairs, an unpaired base on the primer strand re-establishes the normal register. The structural aberration extends to the RNase H active site and may play a role in the resistance of PPT to RNase H cleavage.