Summary information and primary citation
- PDB-id
- 1i7d; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- isomerase-DNA
- Method
- X-ray (2.05 Å)
- Summary
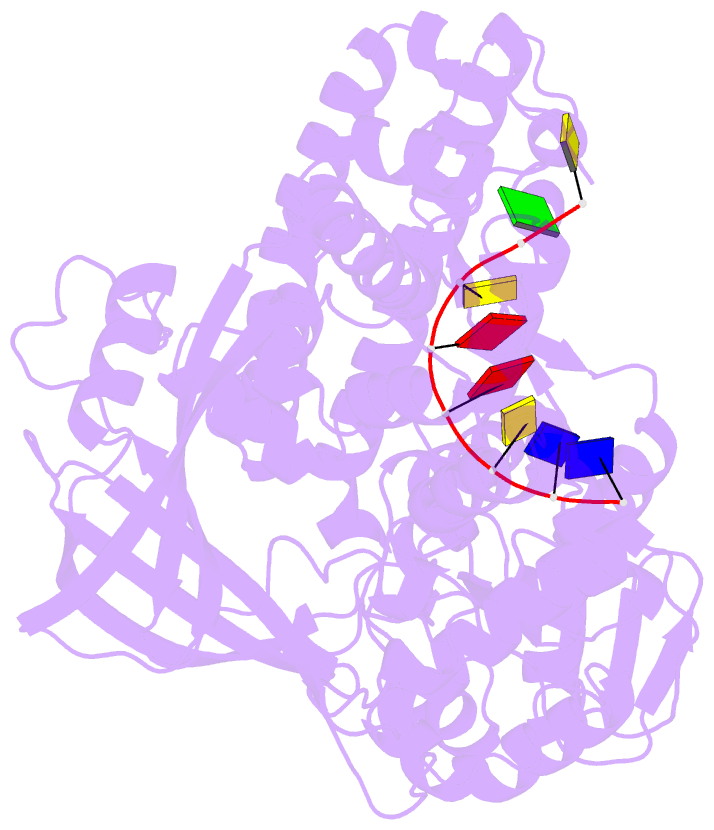
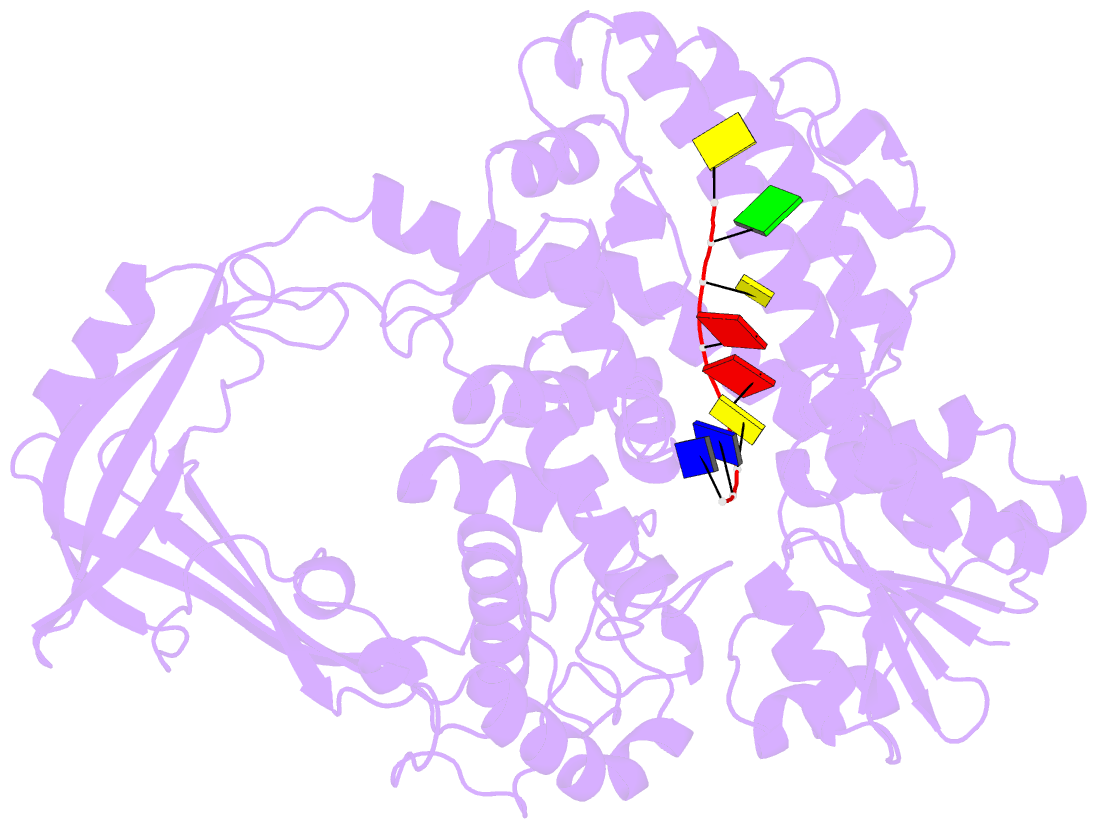
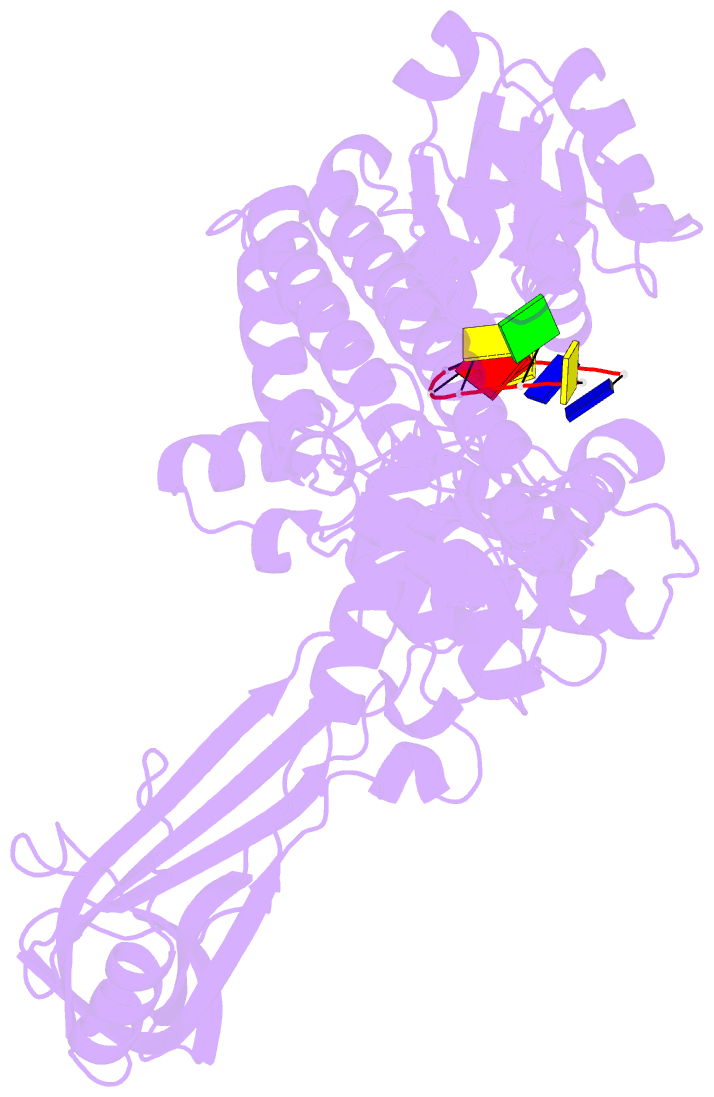
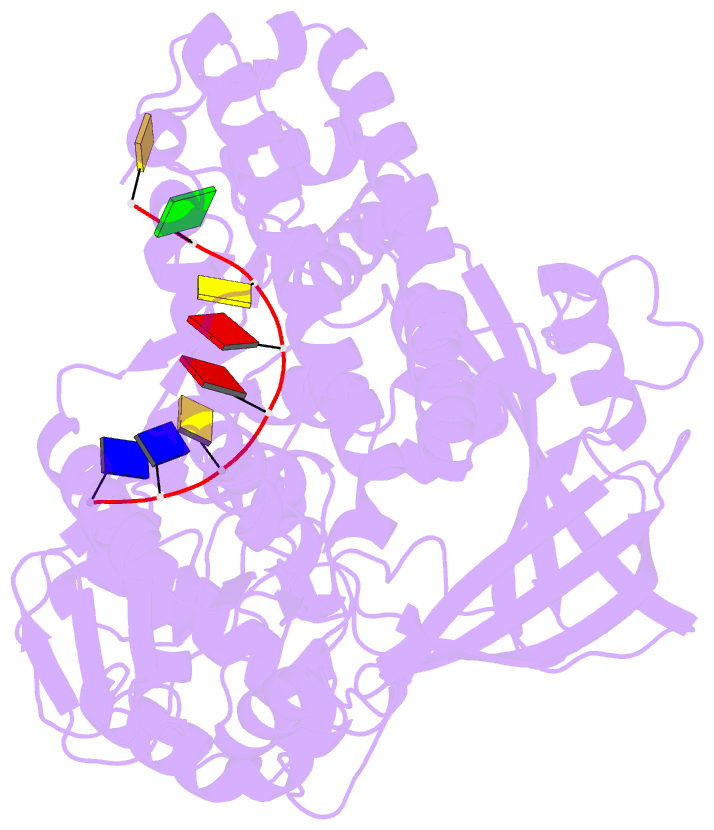
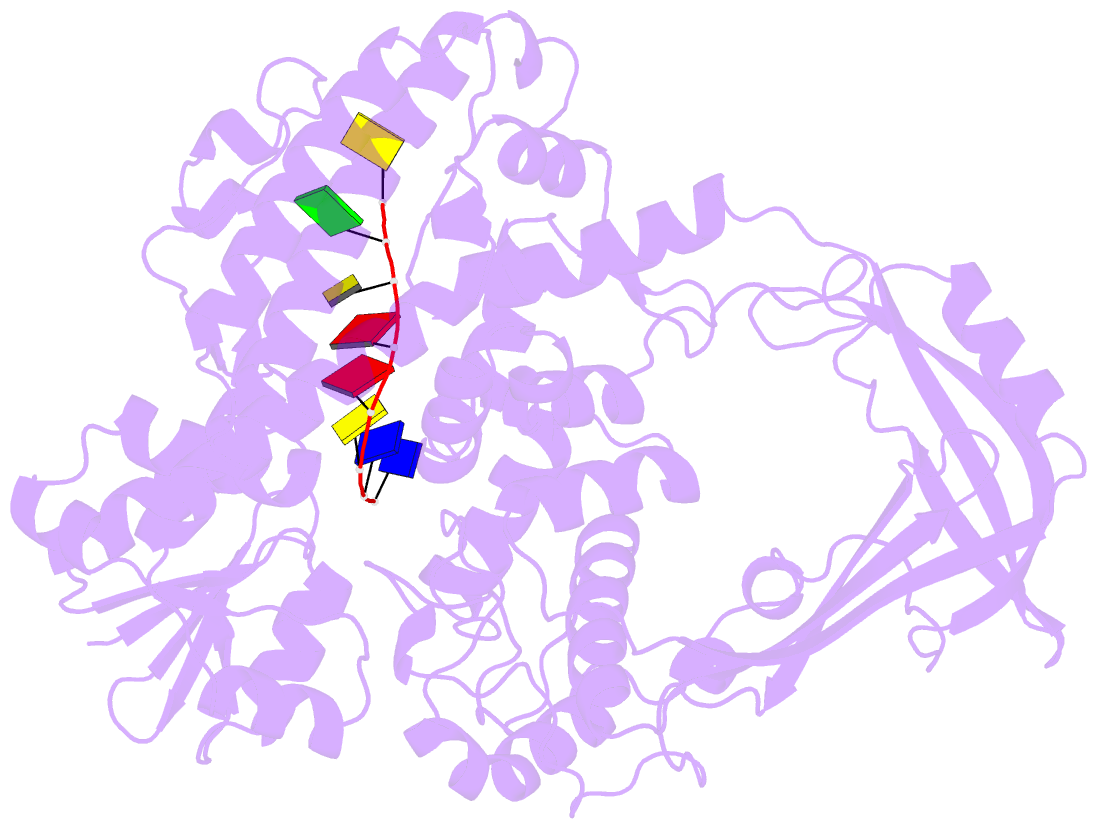
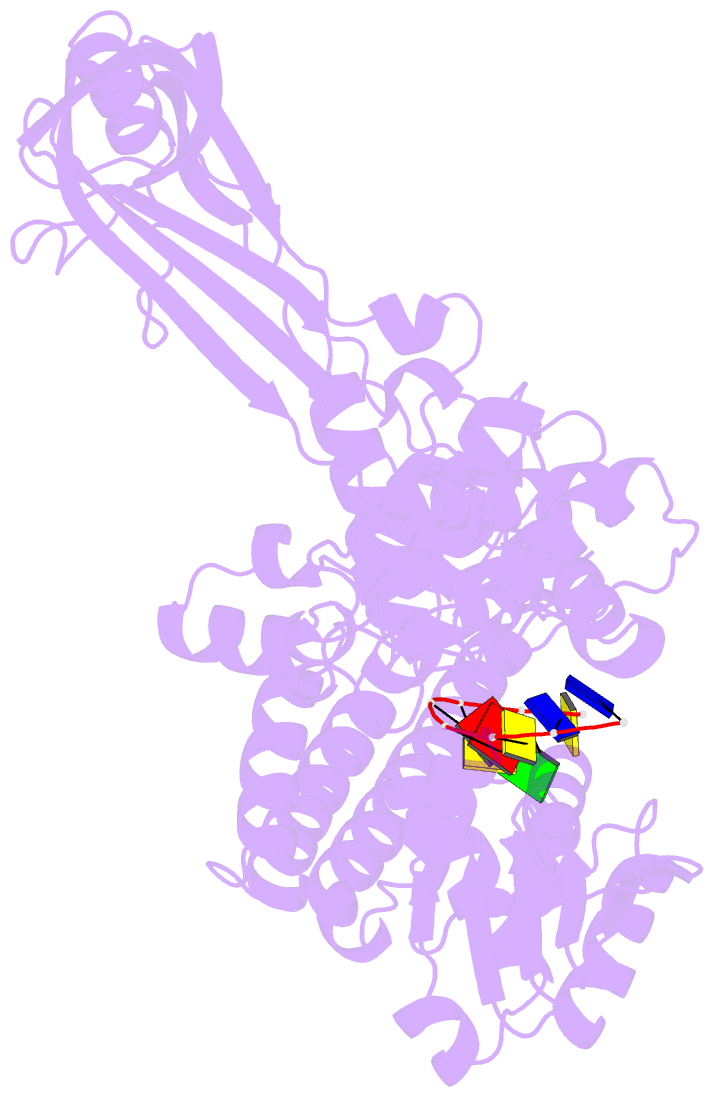
- Noncovalent complex of e.coli DNA topoisomerase iii with an 8-base single-stranded DNA oligonucleotide
- Reference
- Changela A, DiGate RJ, Mondragon A (2001): "Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule." Nature, 411, 1077-1081. doi: 10.1038/35082615.
- Abstract
- A variety of cellular processes, including DNA replication, transcription, and chromosome condensation, require enzymes that can regulate the ensuing topological changes occurring in DNA. Such enzymes-DNA topoisomerases-alter DNA topology by catalysing the cleavage of single-stranded DNA (ssDNA) or double-stranded DNA (dsDNA), the passage of DNA through the resulting break, and the rejoining of the broken phosphodiester backbone. DNA topoisomerase III from Escherichia coli belongs to the type IA family of DNA topoisomerases, which transiently cleave ssDNA via formation of a covalent 5' phosphotyrosine intermediate. Here we report the crystal structure, at 2.05 A resolution, of an inactive mutant of E. coli DNA topoisomerase III in a non-covalent complex with an 8-base ssDNA molecule. The enzyme undergoes a conformational change that allows the oligonucleotide to bind within a groove leading to the active site. We note that the ssDNA molecule adopts a conformation like that of B-DNA while bound to the enzyme. The position of the DNA within the realigned active site provides insight into the role of several highly conserved residues during catalysis. These findings confirm various aspects of the type IA topoisomerase mechanism while suggesting functional implications for other topoisomerases and proteins that perform DNA rearrangements.