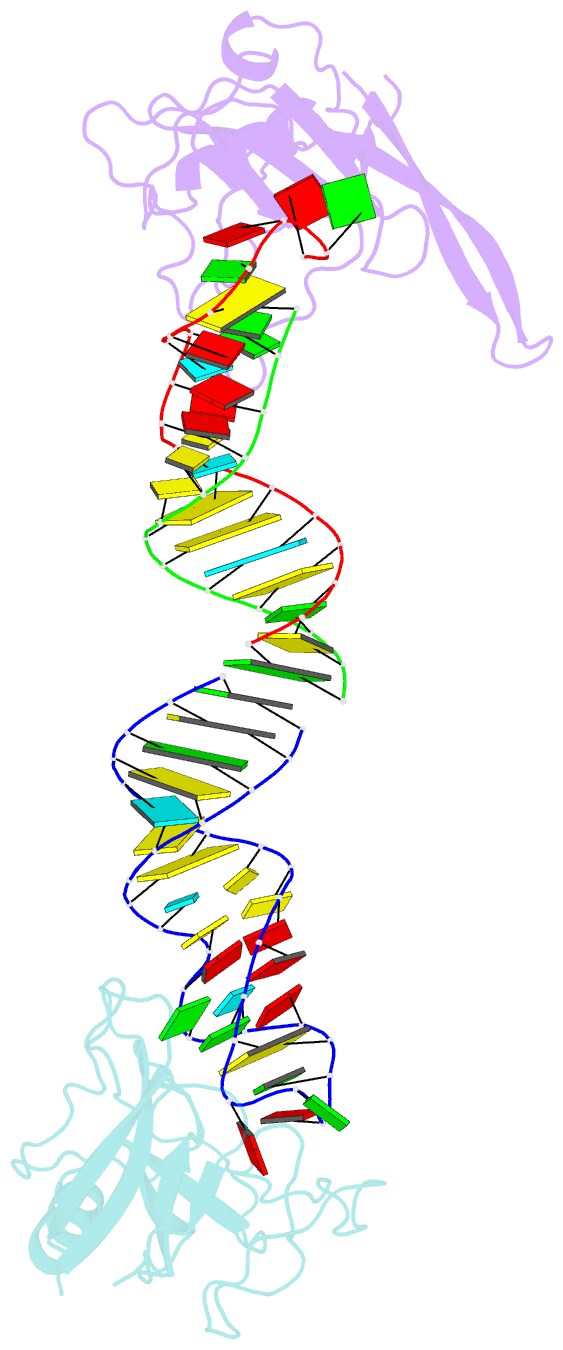
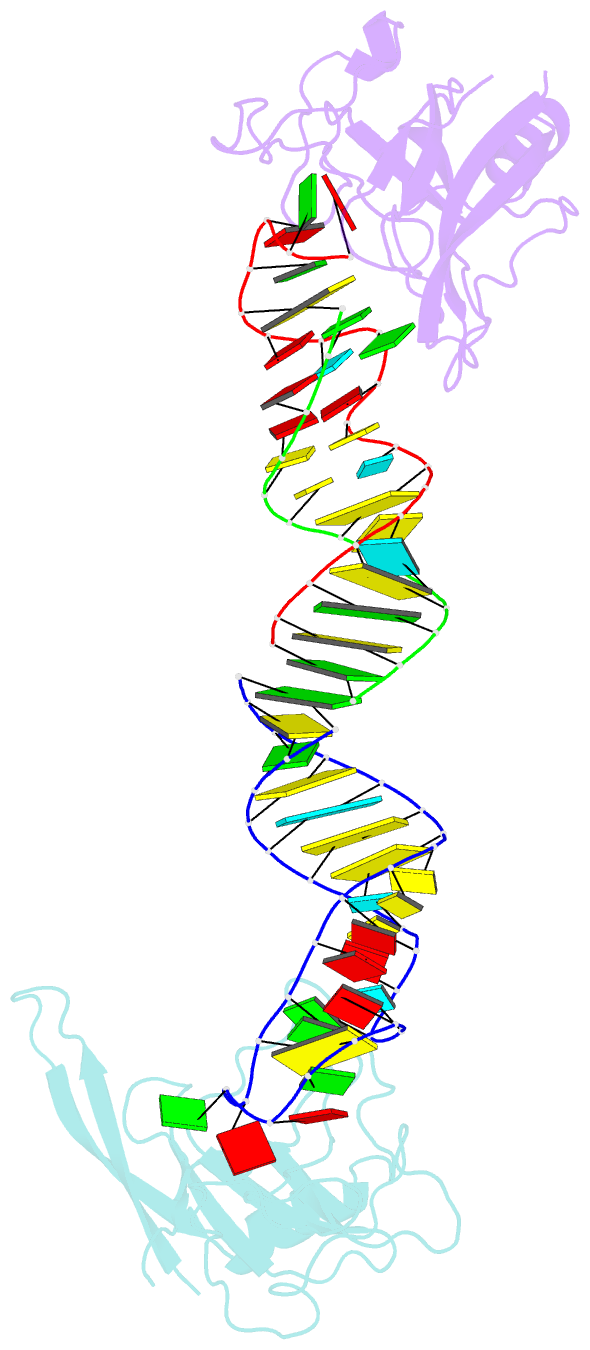
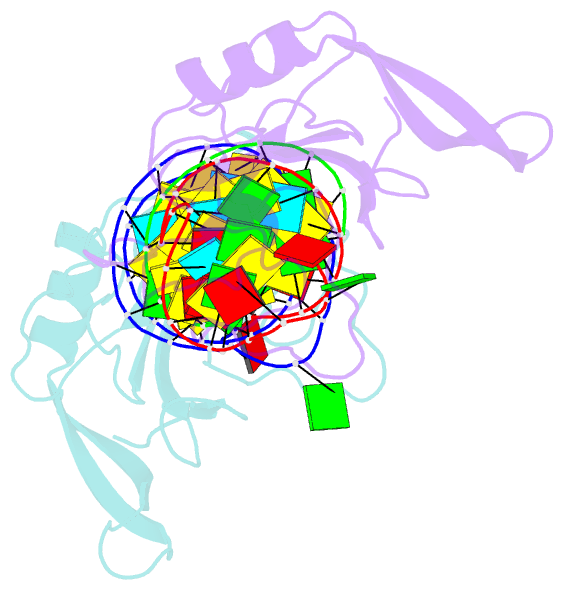
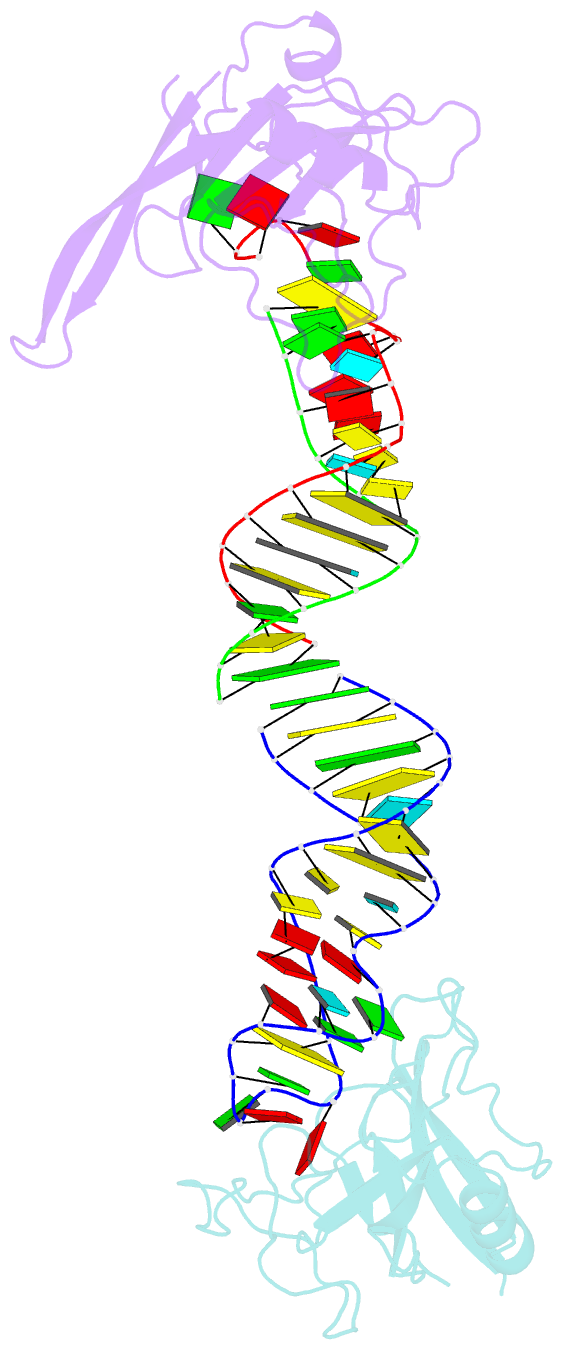
Summary information and primary citation
- PDB-id
-
1jbr;
DSSR-derived features in text and
JSON formats; DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.15 Å)
- Summary
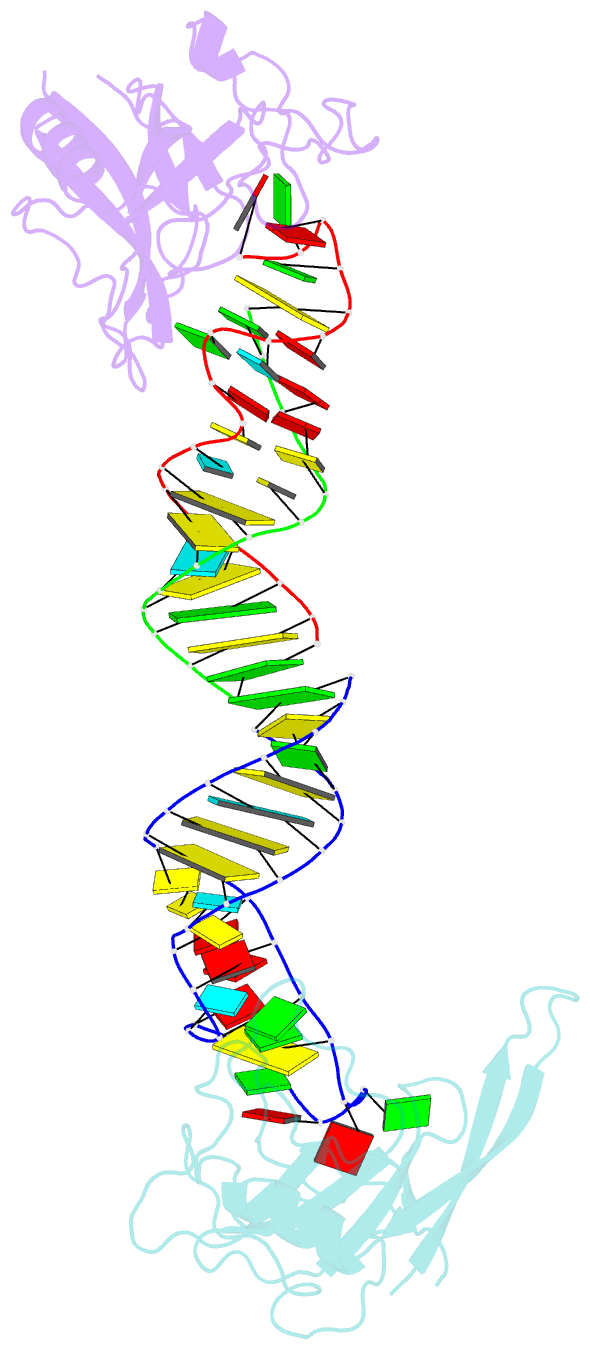
- Crystal structure of the ribotoxin restrictocin and a
31-mer srd RNA inhibitor
- Reference
-
Yang X, Gerczei T, Glover LT, Correll CC (2001):
"Crystal
structures of restrictocin-inhibitor complexes with
implications for RNA recognition and base flipping."
Nat.Struct.Biol., 8, 968-973.
doi: 10.1038/nsb1101-968.
- Abstract
- The cytotoxin sarcin disrupts elongation factor binding
and protein synthesis by specifically cleaving one
phosphodiester bond in ribosomes. To elucidate the
molecular basis of toxin action, we determined three
cocrystal structures of the sarcin homolog restrictocin
bound to different analogs that mimic the target
sarcin/ricin loop (SRL) structure of the rat 28S rRNA. In
these structures, restrictocin contacts the bulged-G motif
and an unfolded form of the tetraloop of the SRL RNA. In
one structure, toxin loops guide selection of the target
site by contacting the base critical for recognition
(G4319) and the surrounding S-shaped backbone. In another
structure, base flipping of the tetraloop enables cleavage
by placing the target nucleotide in the active site with
the nucleophile nearly inline for attack on the scissile
bond. These structures provide the first views of how a
site-specific protein endonuclease recognizes and cleaves a
folded RNA substrate.