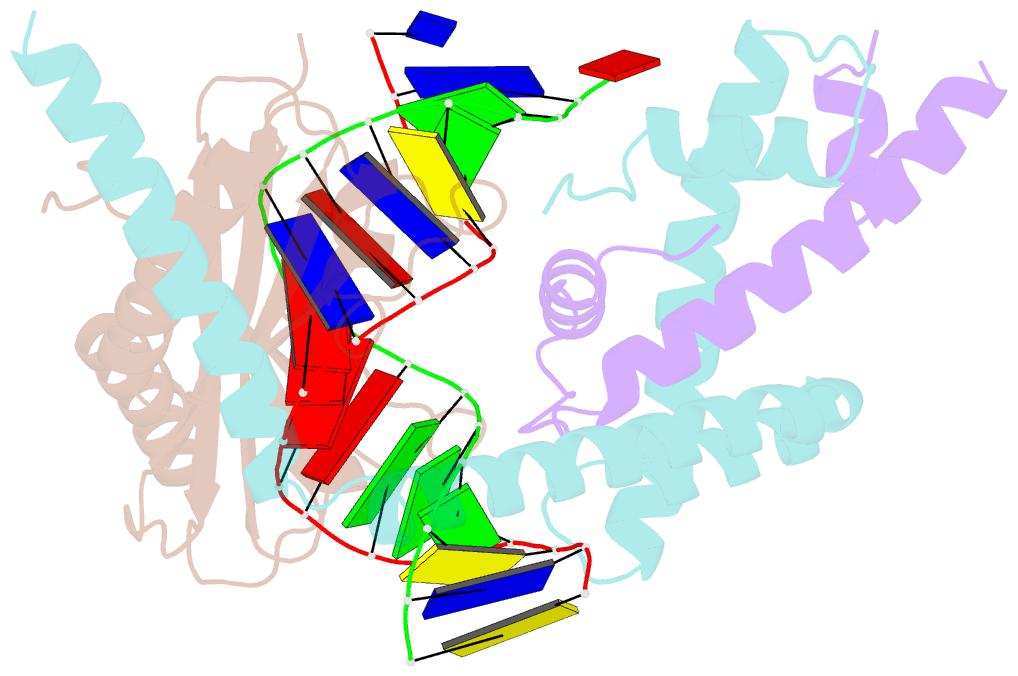
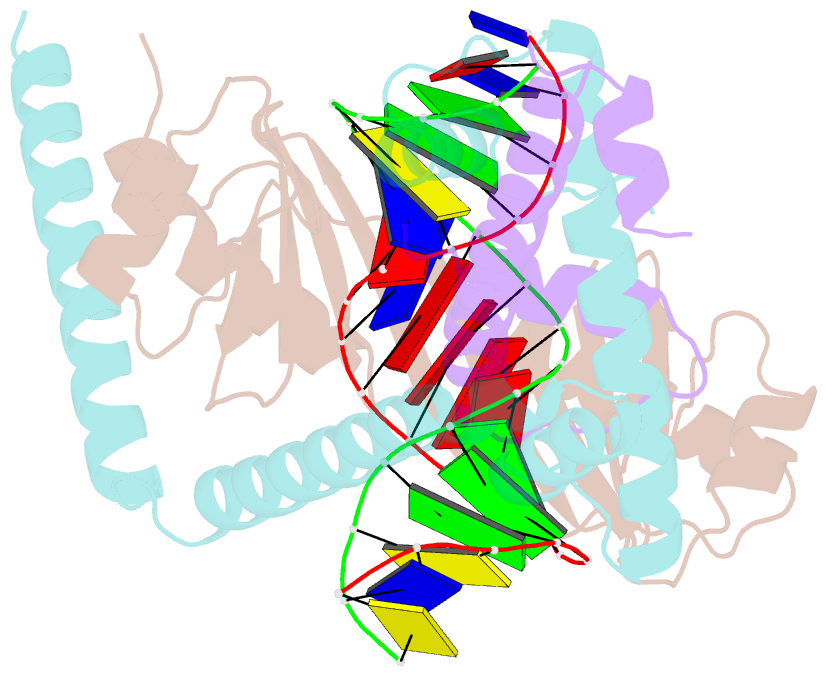
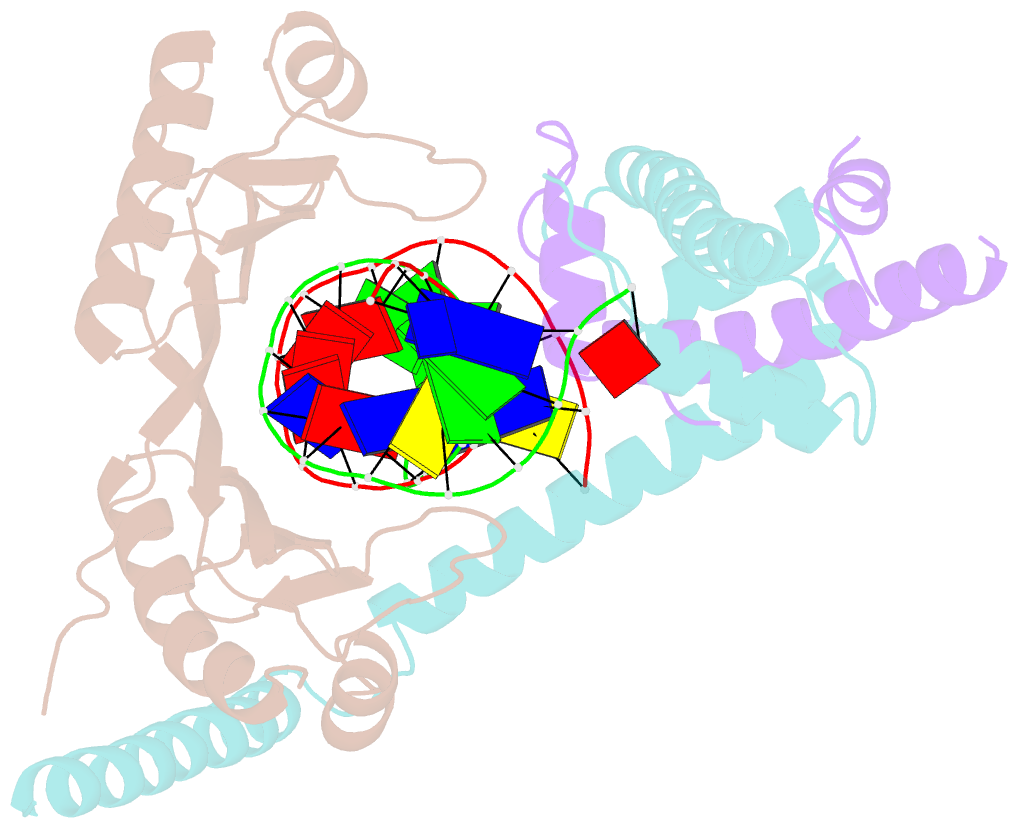
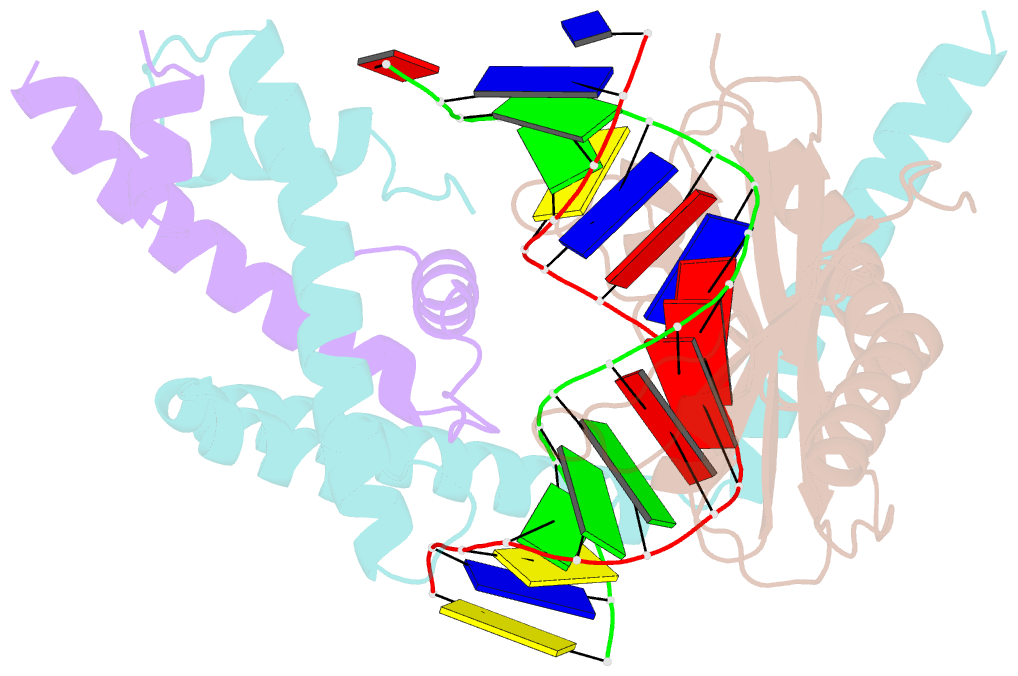
Summary information and primary citation
- PDB-id
- 1jfi; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.62 Å)
- Summary
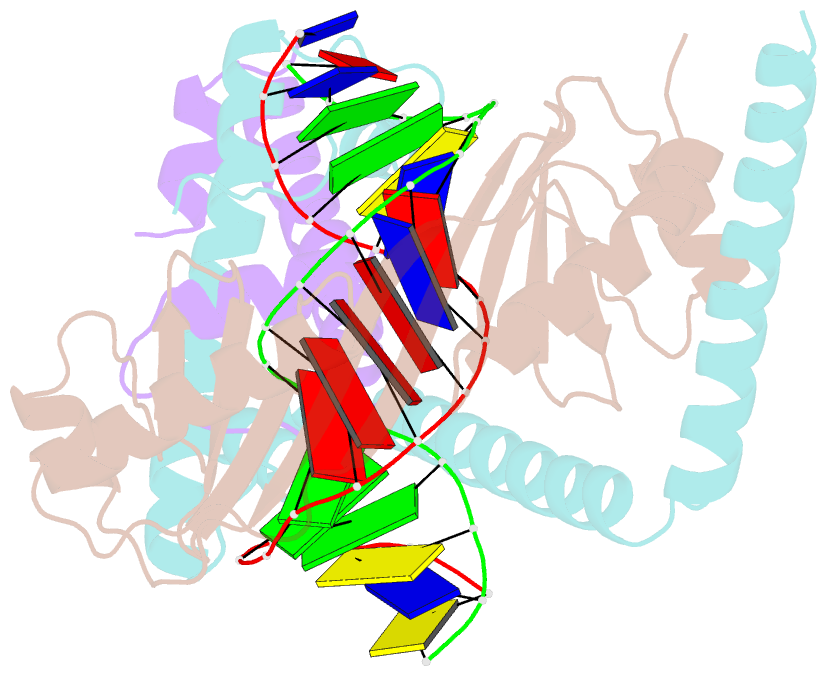
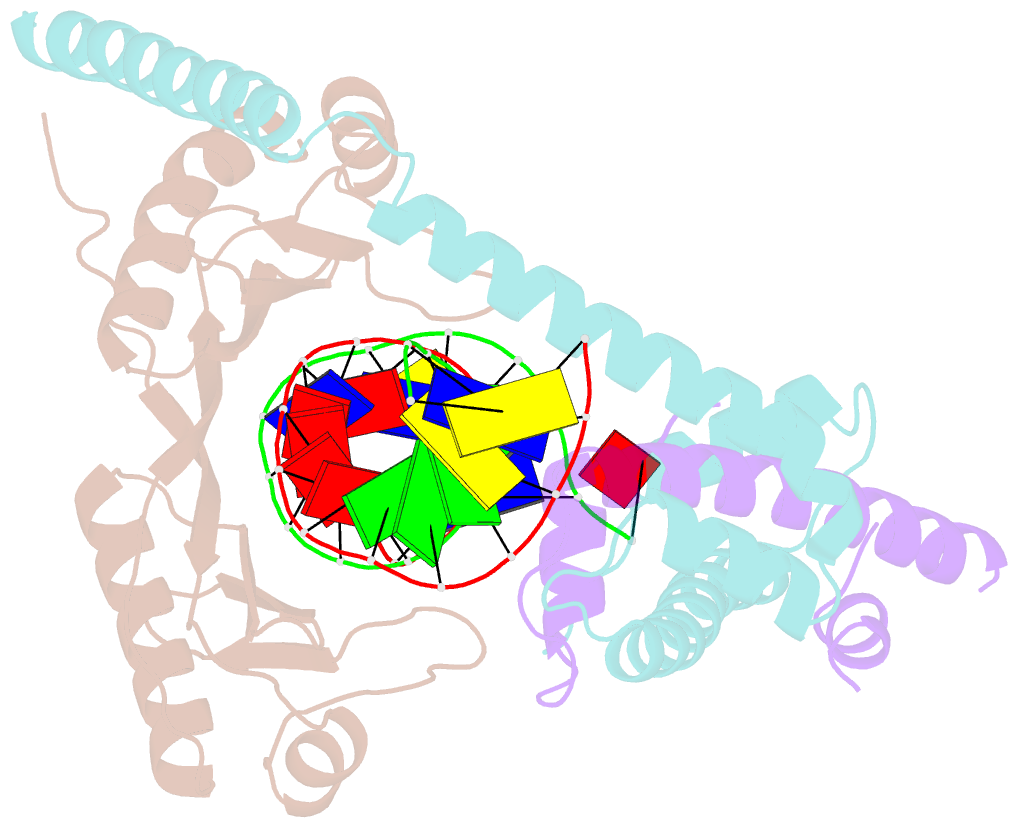
- Crystal structure of the nc2-tbp-DNA ternary complex
- Reference
- Kamada K, Shu F, Chen H, Malik S, Stelzer G, Roeder RG, Meisterernst M, Burley SK (2001): "Crystal structure of negative cofactor 2 recognizing the TBP-DNA transcription complex." Cell(Cambridge,Mass.), 106, 71-81. doi: 10.1016/S0092-8674(01)00417-2.
- Abstract
- The X-ray structure of a ternary complex of Negative Cofactor 2 (NC2), the TATA box binding protein (TBP), and DNA has been determined at 2.6 A resolution. The N termini of NC2 alpha and beta resemble histones H2A and H2B, respectively, and form a heterodimer that binds to the bent DNA double helix on the underside of the preformed TBP-DNA complex via electrostatic interactions. NC2beta contributes to inhibition of TATA-dependent transcription through interactions of its C-terminal alpha helix with a conserved hydrophobic feature on the upper surface of TBP, which in turn positions the penultimate alpha helix of NC2beta to block recognition of the TBP-DNA complex by transcription factor IIB. Further regulatory implications of the NC2 heterodimer structure are discussed.