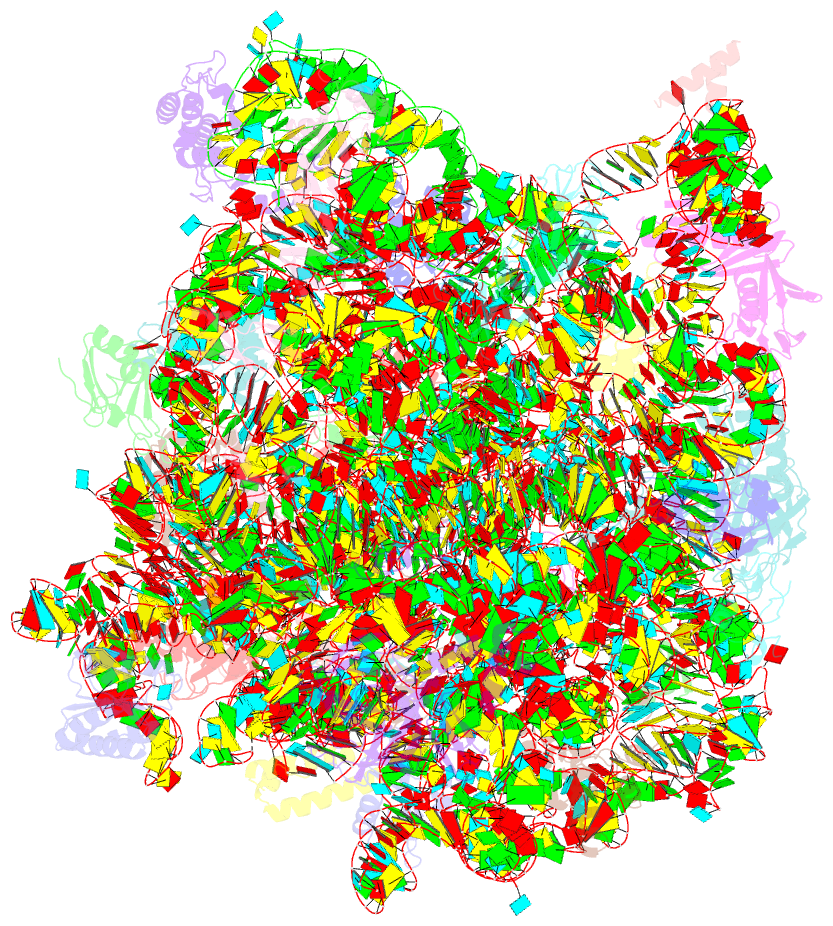
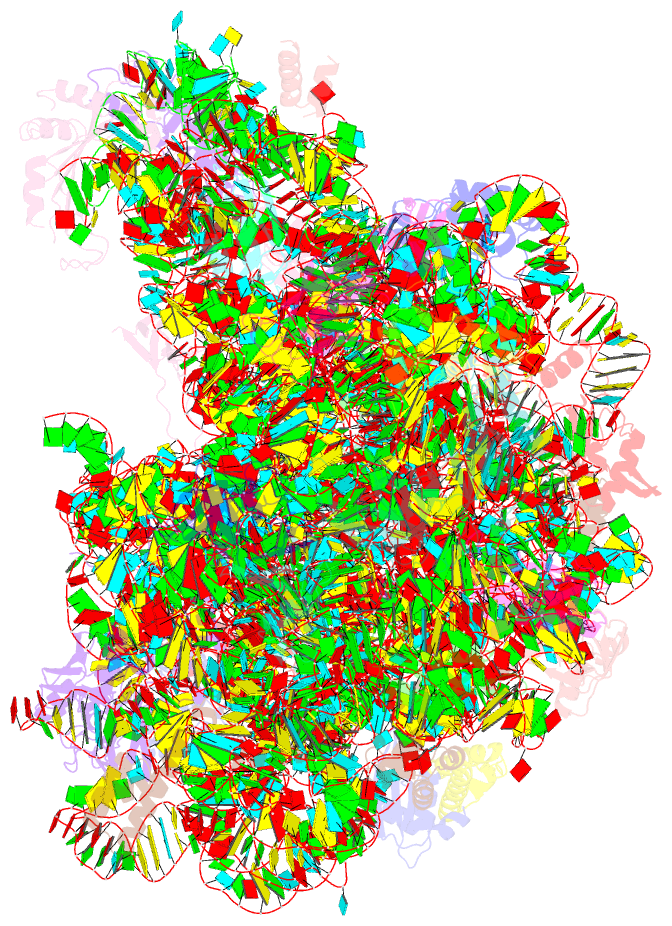
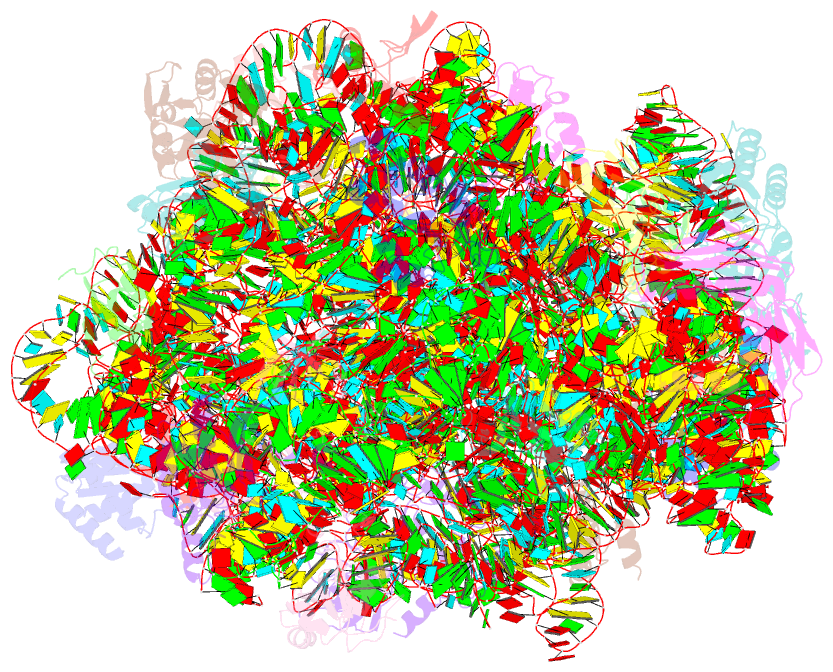
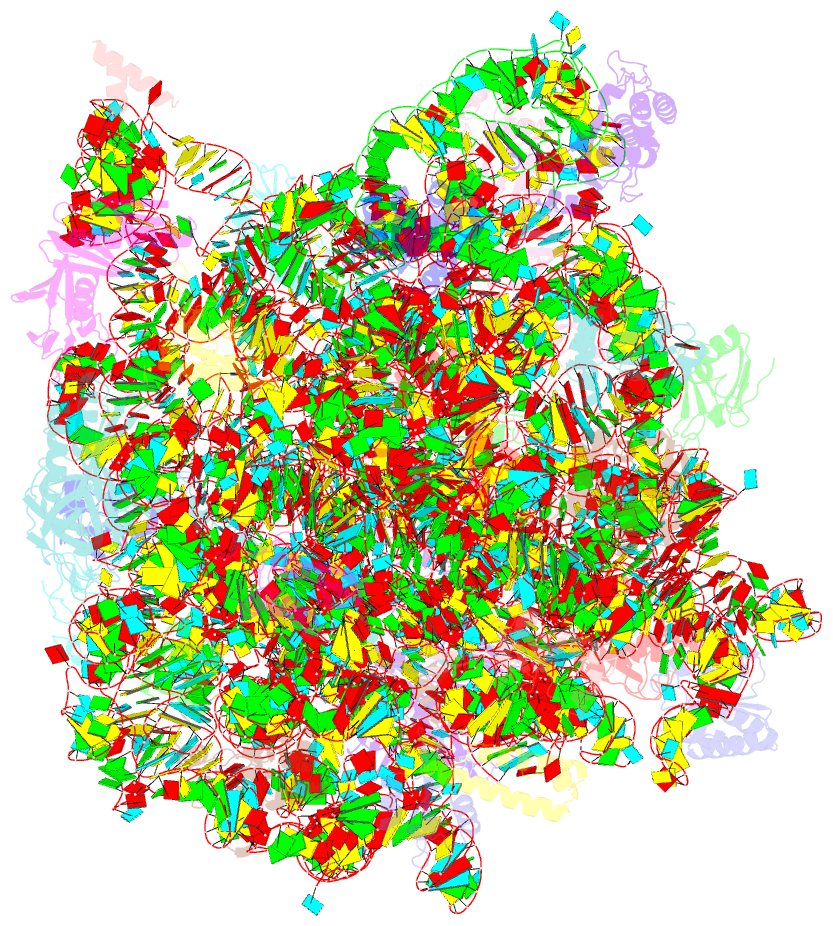
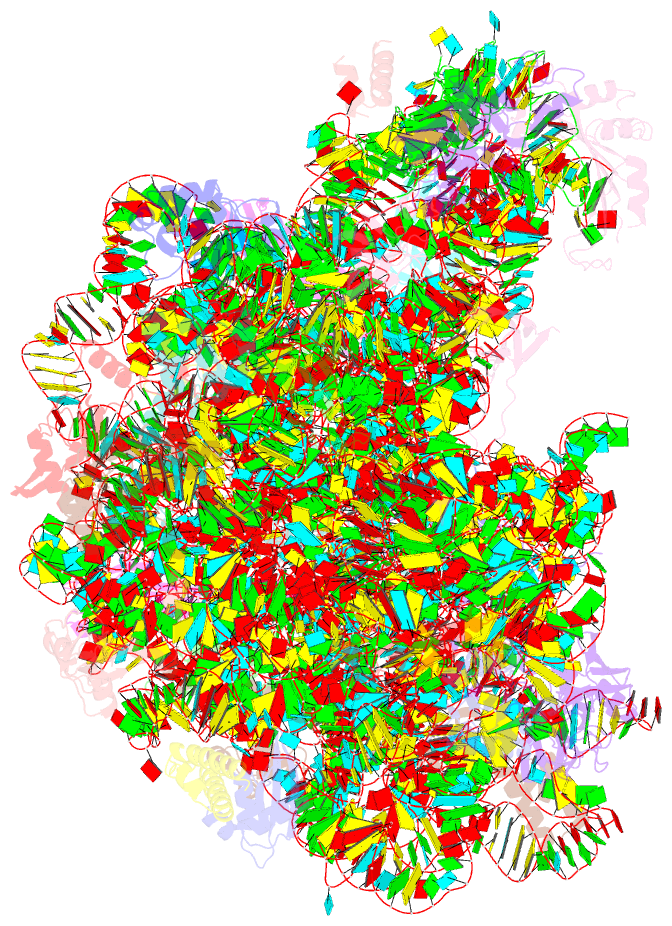
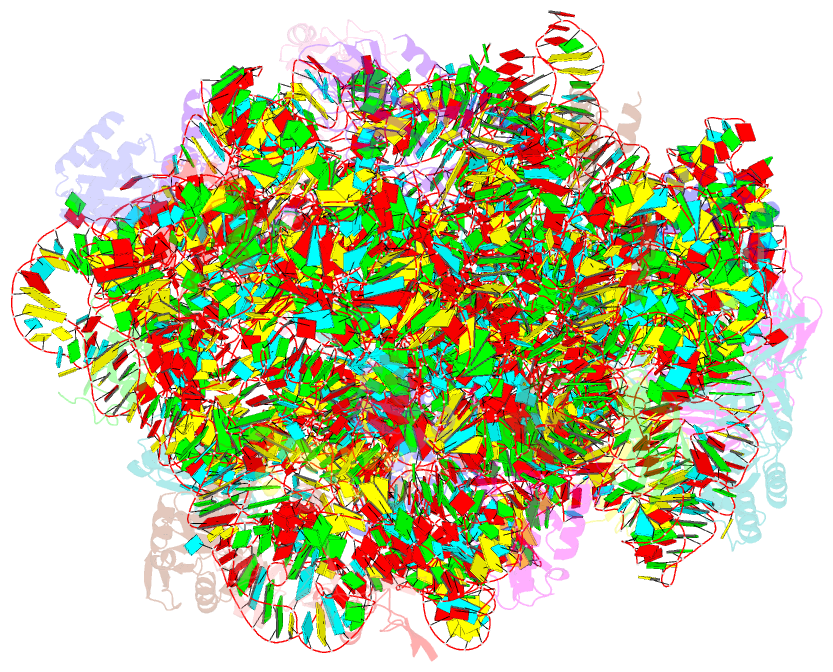
Summary information and primary citation
- PDB-id
- 1jj2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (2.4 Å)
- Summary
- Fully refined crystal structure of the haloarcula marismortui large ribosomal subunit at 2.4 angstrom resolution
- Reference
- Klein DJ, Schmeing TM, Moore PB, Steitz TA (2001): "The kink-turn: a new RNA secondary structure motif." EMBO J., 20, 4214-4221. doi: 10.1093/emboj/20.15.4214.
- Abstract
- Analysis of the Haloarcula marismortui large ribosomal subunit has revealed a common RNA structure that we call the kink-turn, or K-turn. The six K-turns in H.marismortui 23S rRNA superimpose with an r.m.s.d. of 1.7 A. There are two K-turns in the structure of Thermus thermophilus 16S rRNA, and the structures of U4 snRNA and L30e mRNA fragments form K-turns. The structure has a kink in the phosphodiester backbone that causes a sharp turn in the RNA helix. Its asymmetric internal loop is flanked by C-G base pairs on one side and sheared G-A base pairs on the other, with an A-minor interaction between these two helical stems. A derived consensus secondary structure for the K-turn includes 10 consensus nucleotides out of 15, and predicts its presence in the 5'-UTR of L10 mRNA, helix 78 in Escherichia coli 23S rRNA and human RNase MRP. Five K-turns in 23S rRNA interact with nine proteins. While the observed K-turns interact with proteins of unrelated structures in different ways, they interact with L7Ae and two homologous proteins in the same way.