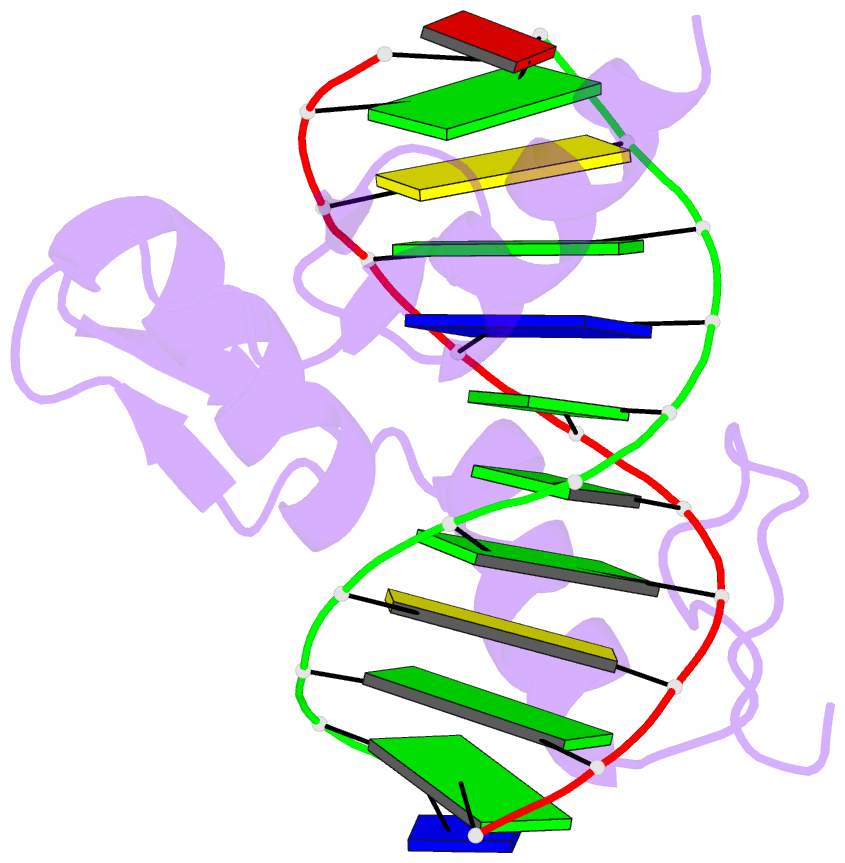
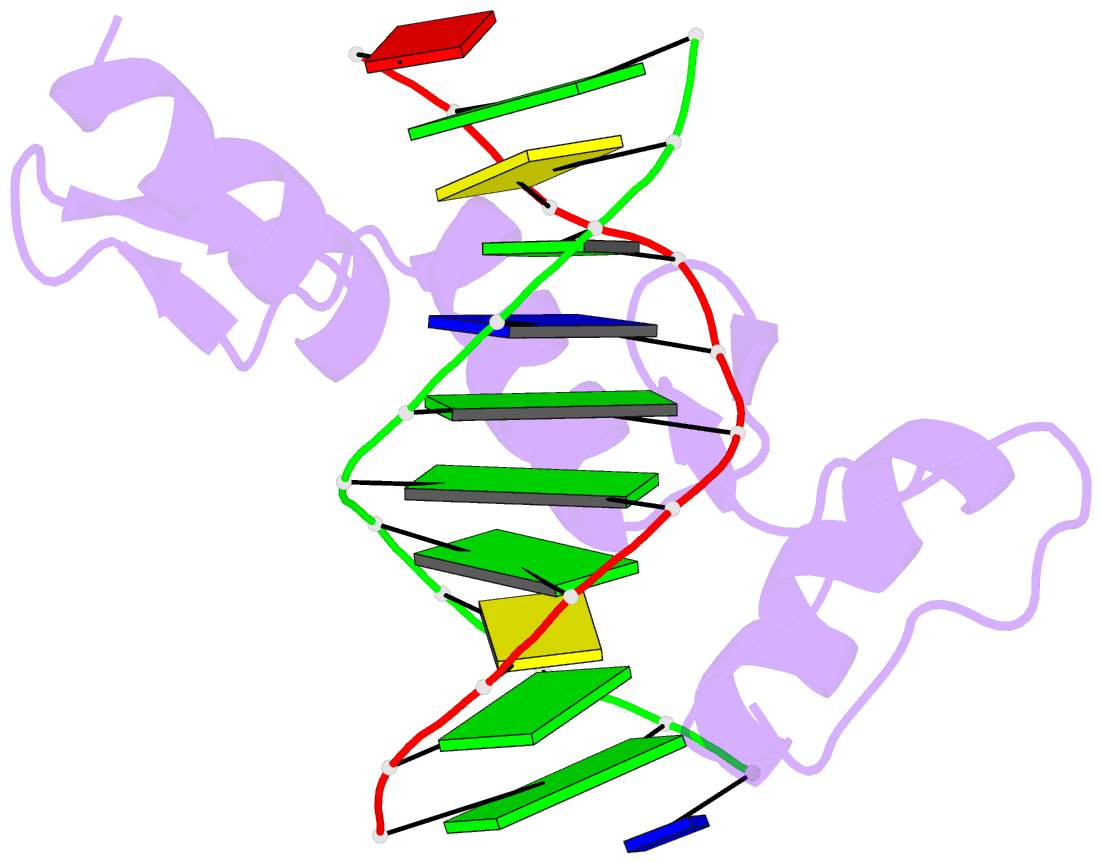
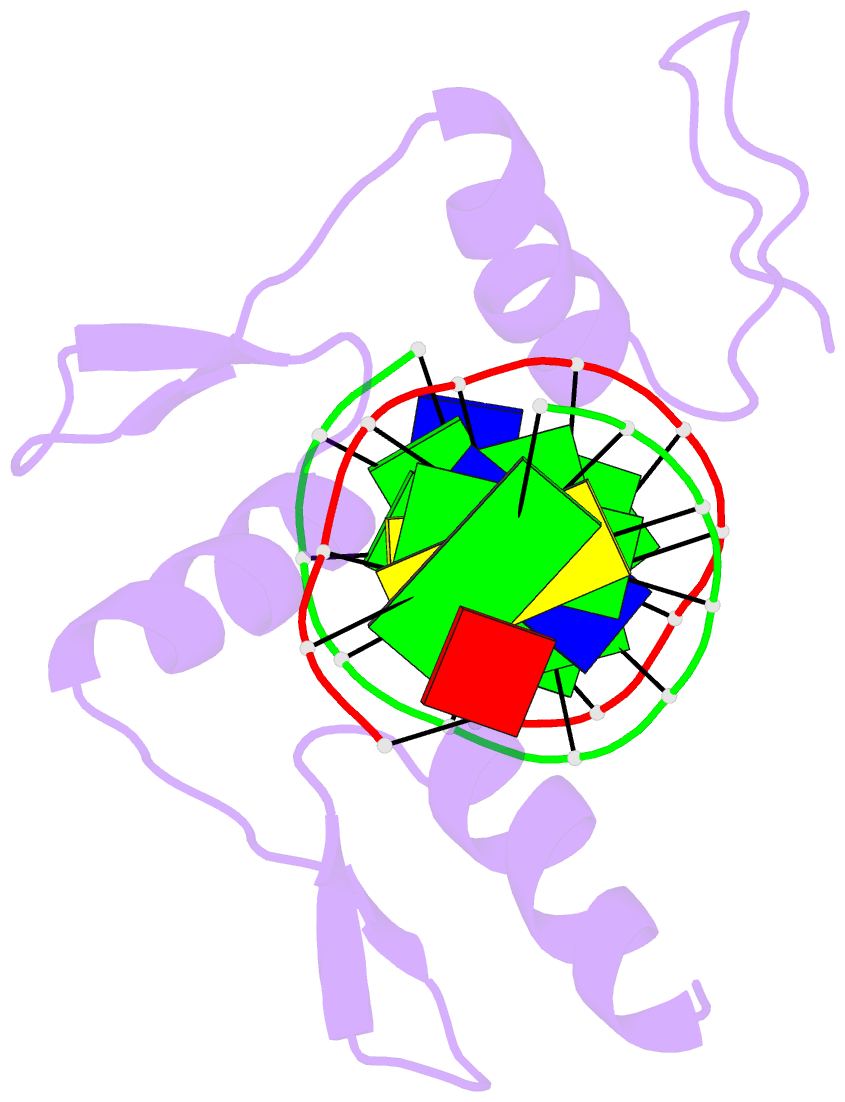
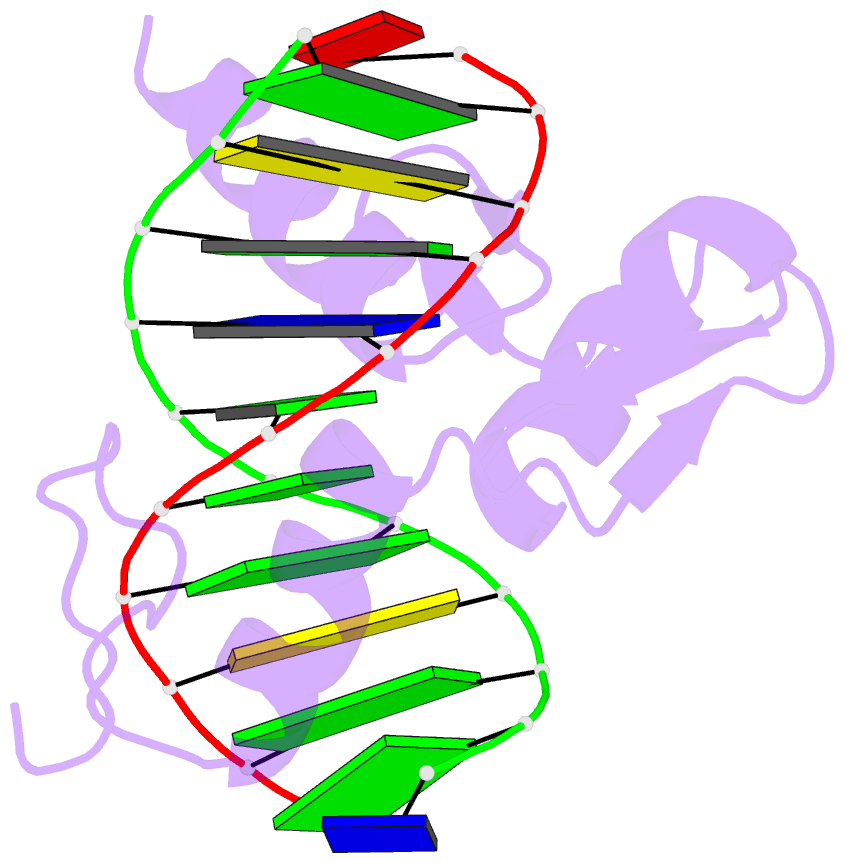
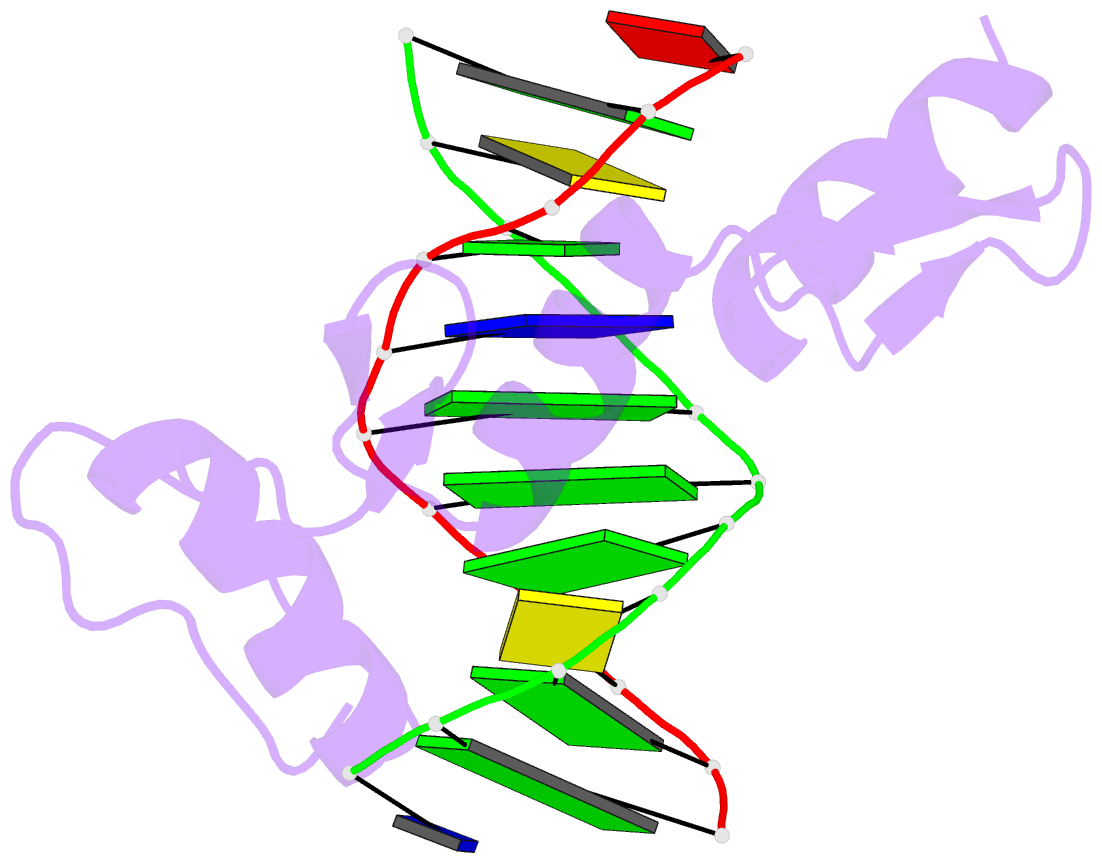
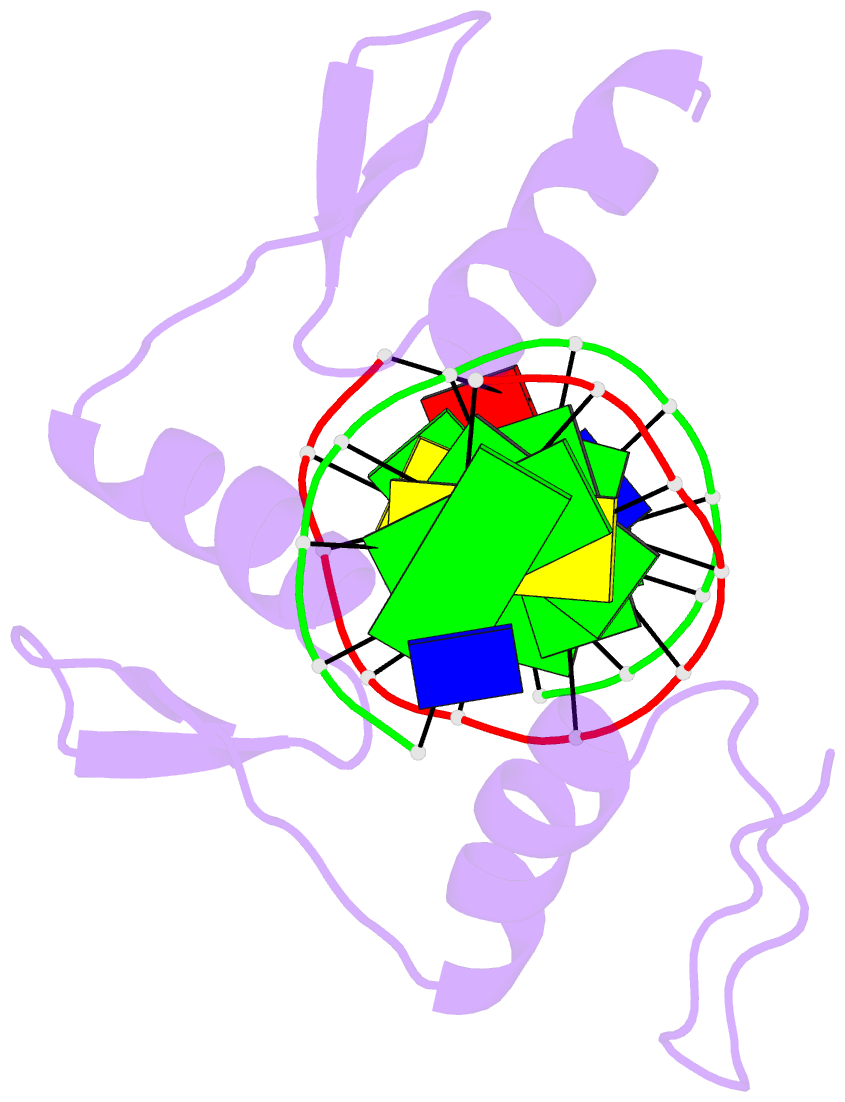
Summary information and primary citation
- PDB-id
- 1jk1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (1.9 Å)
- Summary
- Zif268 d20a mutant bound to wt DNA site
- Reference
- Miller JC, Pabo CO (2001): "Rearrangement of side-chains in a Zif268 mutant highlights the complexities of zinc finger-DNA recognition." J.Mol.Biol., 313, 309-315. doi: 10.1006/jmbi.2001.4975.
- Abstract
- Structural and biochemical studies of Cys(2)His(2) zinc finger proteins initially led several groups to propose a "recognition code" involving a simple set of rules relating key amino acid residues in the zinc finger protein to bases in its DNA site. One recent study from our group, involving geometric analysis of protein-DNA interactions, has discussed limitations of this idea and has shown how the spatial relationship between the polypeptide backbone and the DNA helps to determine what contacts are possible at any given position in a protein-DNA complex. Here we report a study of a zinc finger variant that highlights yet another source of complexity inherent in protein-DNA recognition. In particular, we find that mutations can cause key side-chains to rearrange at the protein-DNA interface without fundamental changes in the spatial relationship between the polypeptide backbone and the DNA. This is clear from a simple analysis of the binding site preferences and co-crystal structures for the Asp20-->Ala point mutant of Zif268. This point mutation in finger one changes the specificity of the protein from GCG TGG GCG to GCG TGG GC(G/T), and we have solved crystal structures of the D20A mutant bound to both types of sites. The structure of the D20A mutant bound to the GCG site reveals that contacts from key residues in the recognition helix are coupled in complex ways. The structure of the complex with the GCT site also shows an important new water molecule at the protein-DNA interface. These side-chain/side-chain interactions, and resultant changes in hydration at the interface, affect binding specificity in ways that cannot be predicted either from a simple recognition code or from analysis of spatial relationships at the protein-DNA interface. Accurate computer modeling of protein-DNA interfaces remains a challenging problem and will require systematic strategies for modeling side-chain rearrangements and change in hydration.