Summary information and primary citation
- PDB-id
- 1kog; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (3.5 Å)
- Summary
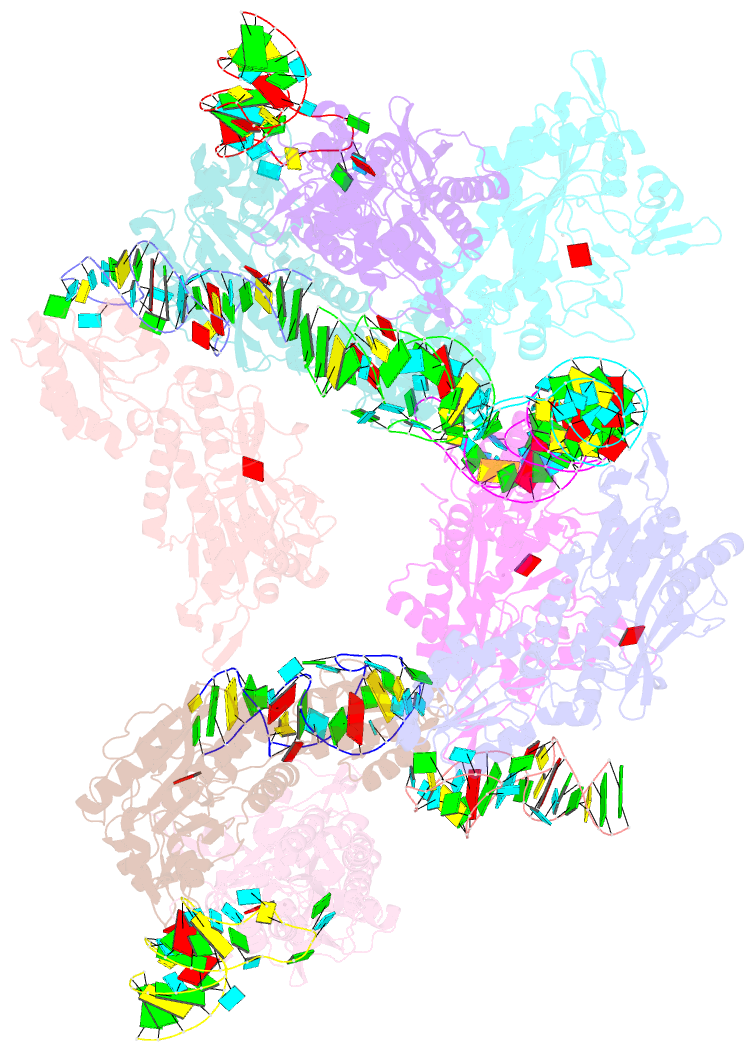
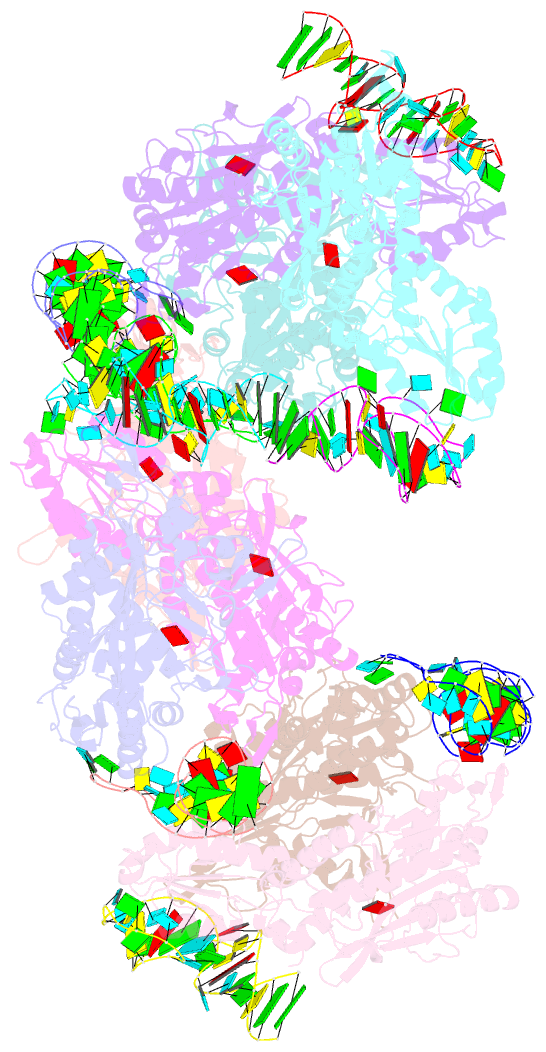
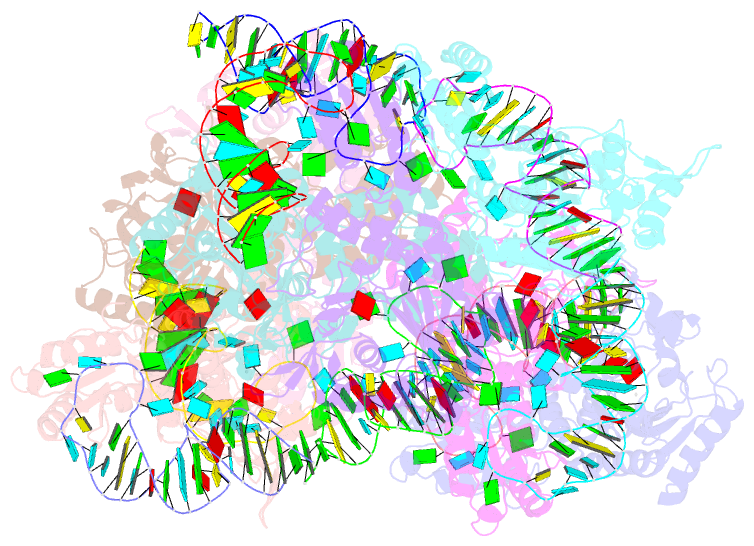
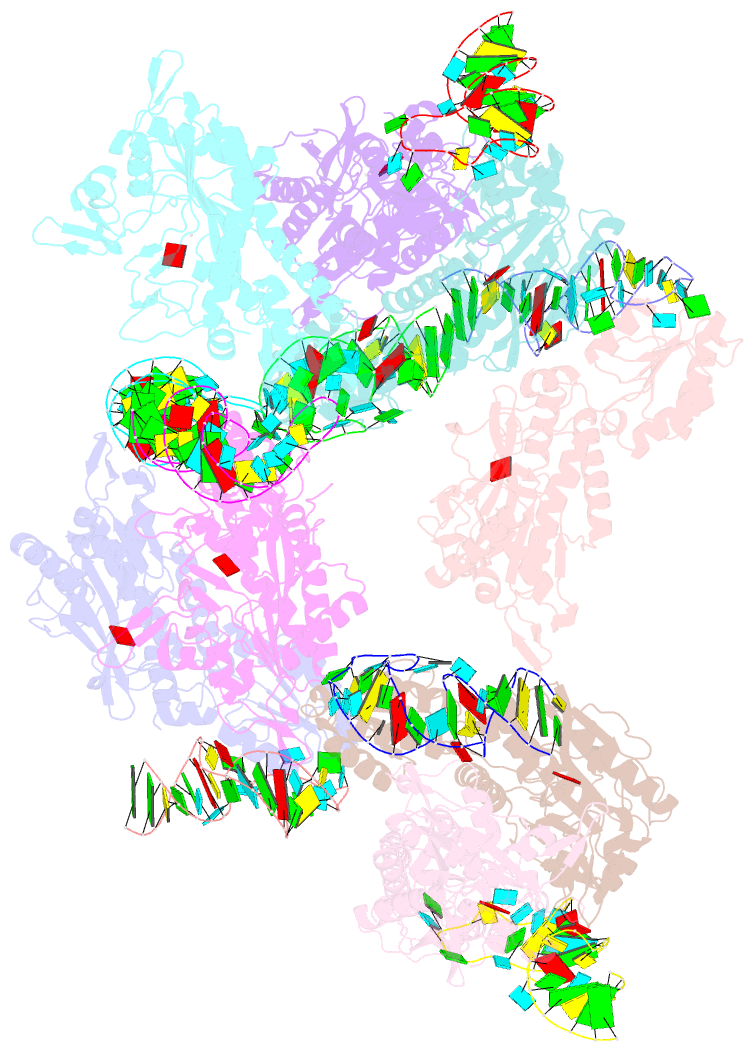
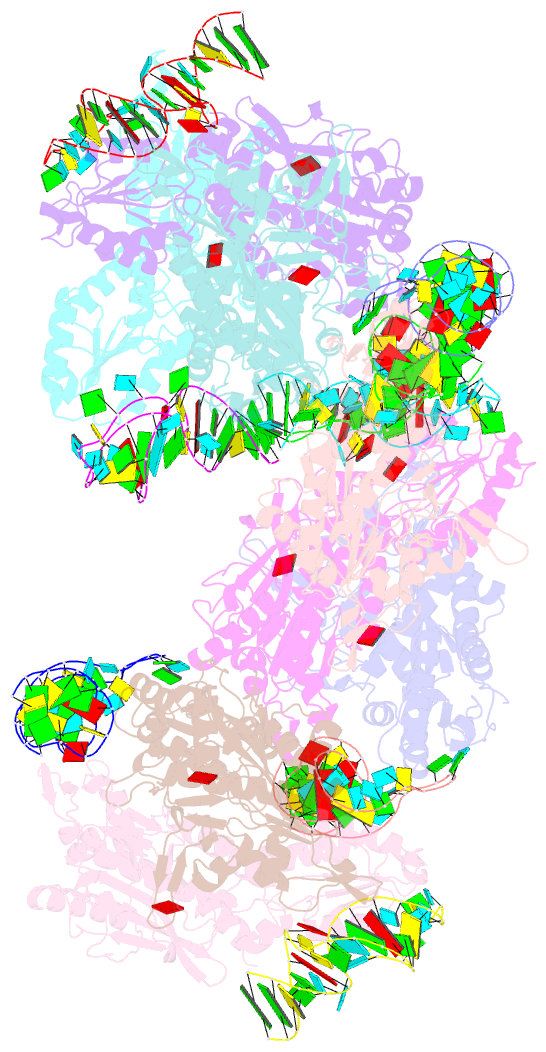
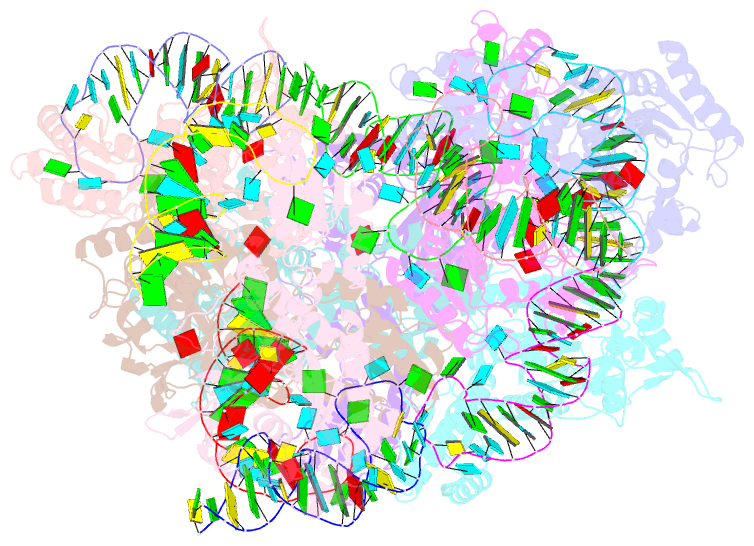
- Crystal structure of e. coli threonyl-trna synthetase interacting with the essential domain of its mrna operator
- Reference
- Torres-Larios A, Dock-Bregeon AC, Romby P, Rees B, Sankaranarayanan R, Caillet J, Springer M, Ehresmann C, Ehresmann B, Moras D (2002): "Structural basis of translational control by Escherichia coli threonyl tRNA synthetase." Nat.Struct.Biol., 9, 343-347.
- Abstract
- Escherichia coli threonyl-tRNA synthetase (ThrRS) represses the translation of its own messenger RNA by binding to an operator located upstream of the initiation codon. The crystal structure of the complex between the core of ThrRS and the essential domain of the operator shows that the mRNA uses the recognition mode of the tRNA anticodon loop to initiate binding. The final positioning of the operator, upon which the control mechanism is based, relies on a characteristic RNA motif adapted to the enzyme surface. The finding of other thrS operators that have this conserved motif leads to a generalization of this regulatory mechanism to a subset of Gram-negative bacteria.