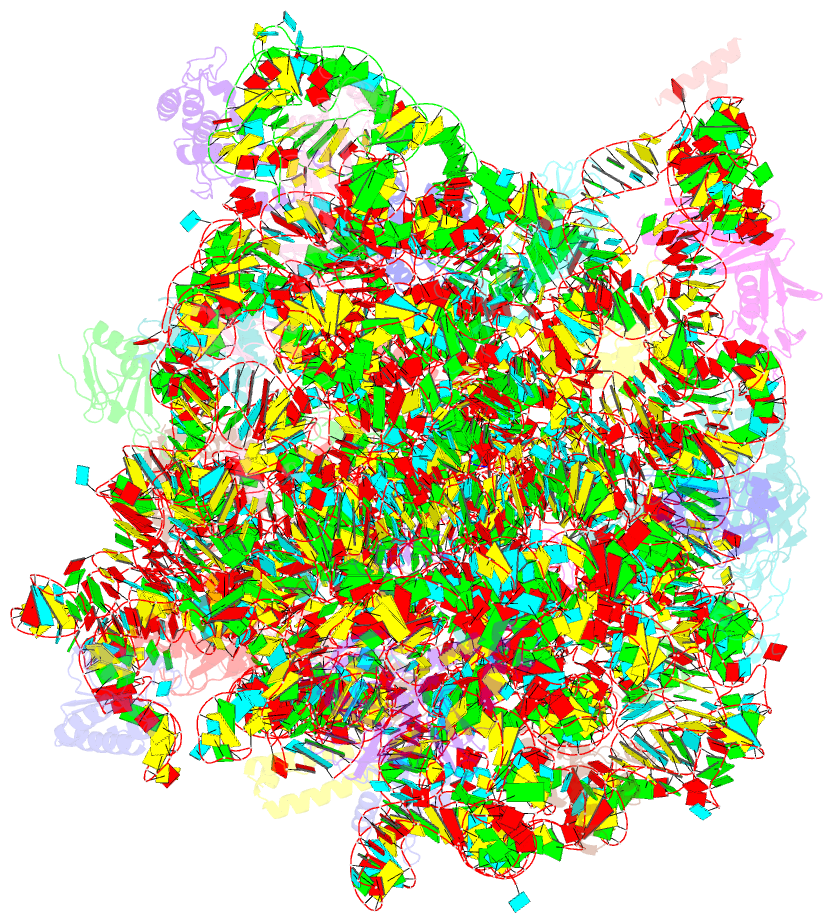
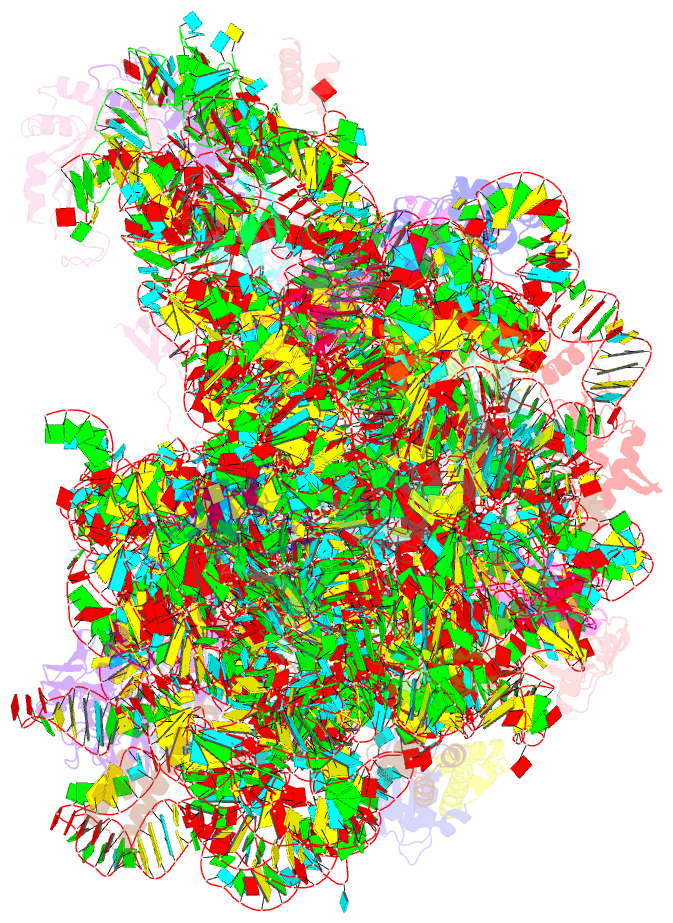
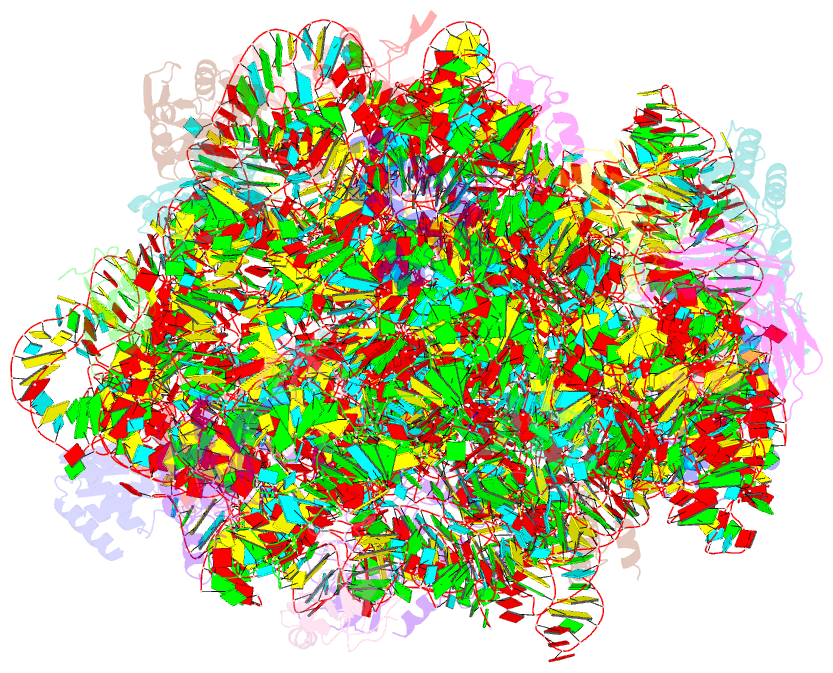
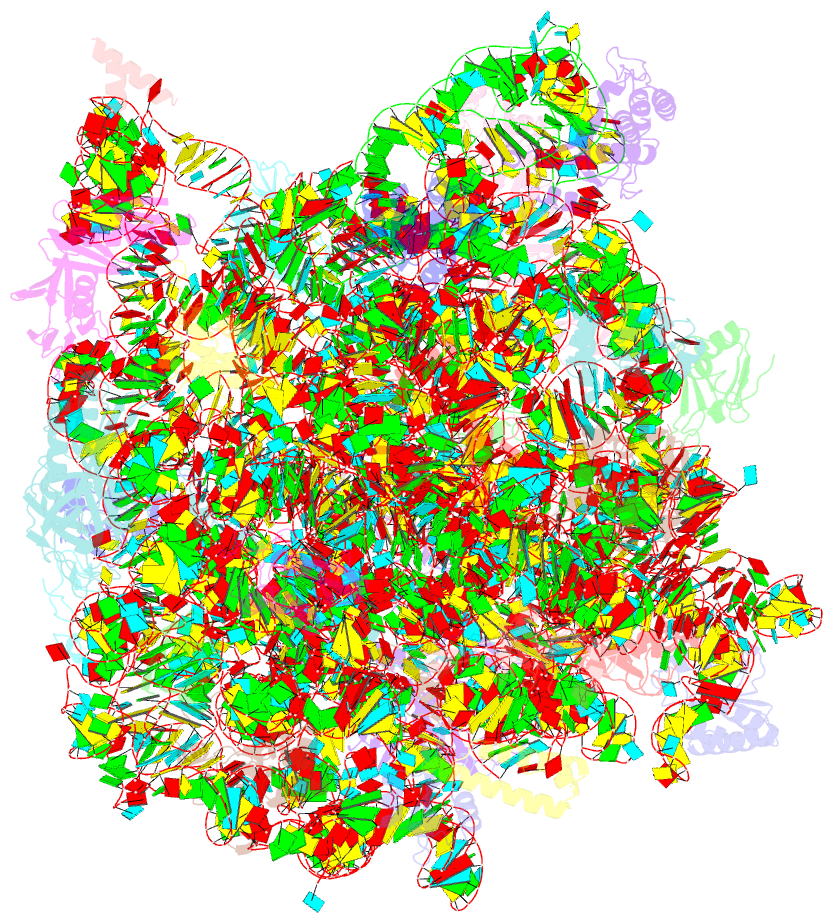
Summary information and primary citation
- PDB-id
- 1kqs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (3.1 Å)
- Summary
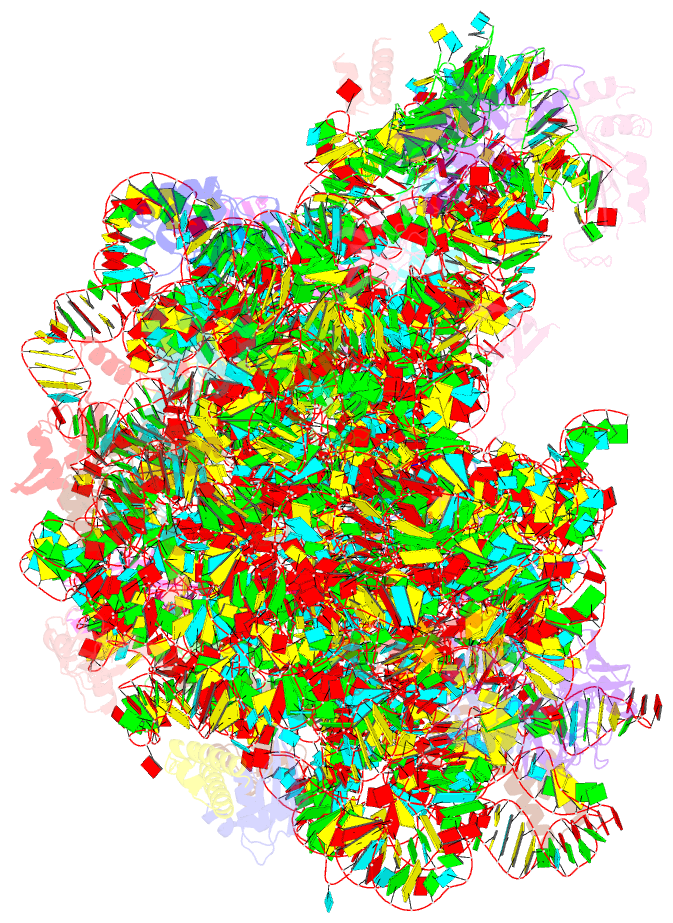
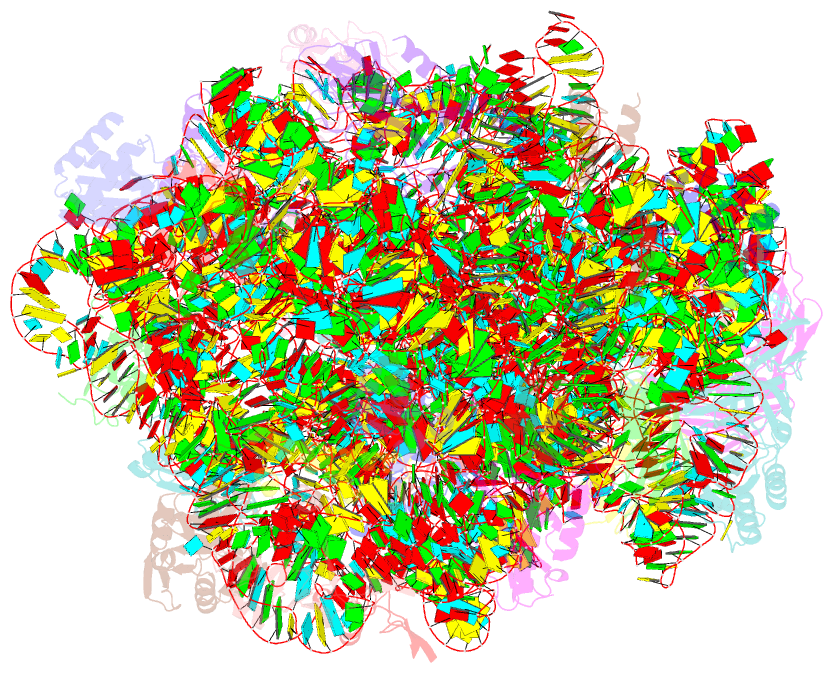
- The haloarcula marismortui 50s complexed with a pretranslocational intermediate in protein synthesis
- Reference
- Schmeing TM, Seila AC, Hansen JL, Freeborn B, Soukup JK, Scaringe SA, Strobel SA, Moore PB, Steitz TA (2002): "A pre-translocational intermediate in protein synthesis observed in crystals of enzymatically active 50S subunits." Nat.Struct.Biol., 9, 225-230.
- Abstract
- The large ribosomal subunit catalyzes peptide bond formation during protein synthesis. Its peptidyl transferase activity has often been studied using a 'fragment assay' that depends on high concentrations of methanol or ethanol. Here we describe a version of this assay that does not require alcohol and use it to show, both crystallographically and biochemically, that crystals of the large ribosomal subunits from Haloarcula marismortui are enzymatically active. Addition of these crystals to solutions containing substrates results in formation of products, which ceases when crystals are removed. When substrates are diffused into large subunit crystals, the subsequent structure shows that products have formed. The CC-puromycin-peptide product is found bound to the A-site and the deacylated CCA is bound to the P-site, with its 3prime prime or minute OH near N3 A2486 (Escherichia coli A2451). Thus, this structure represents a state that occurs after peptide bond formation but before the hybrid state of protein synthesis.