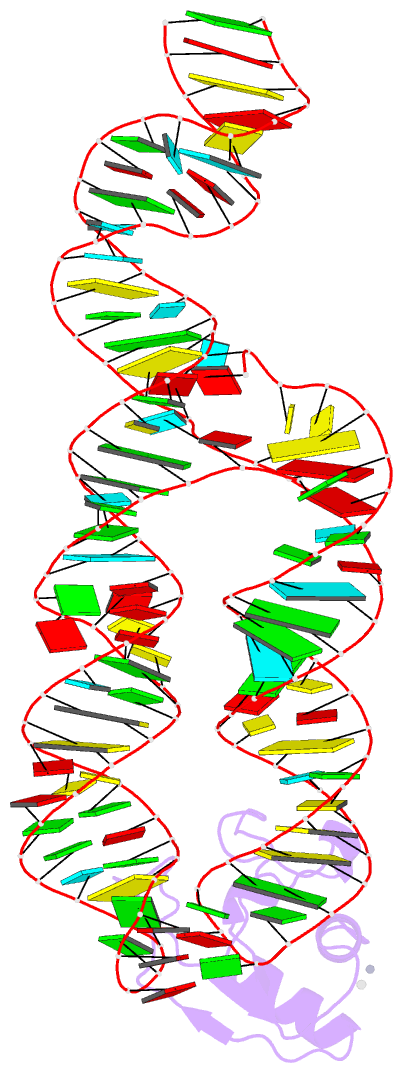
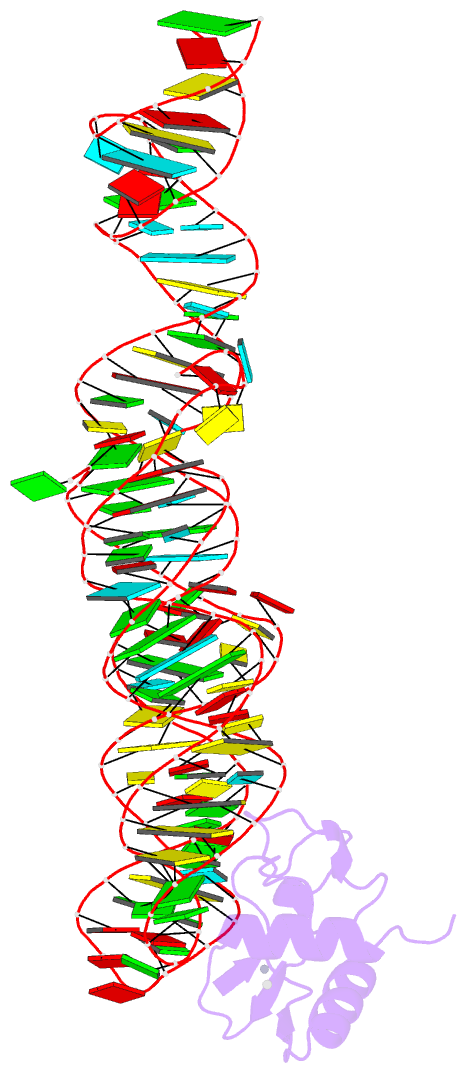
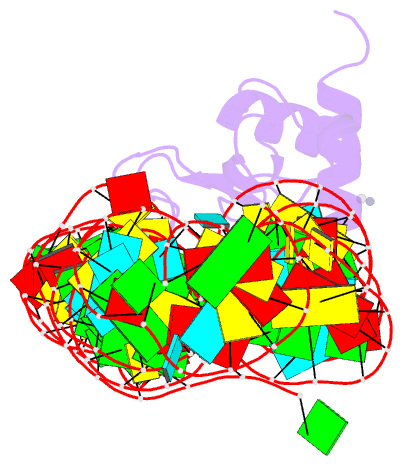
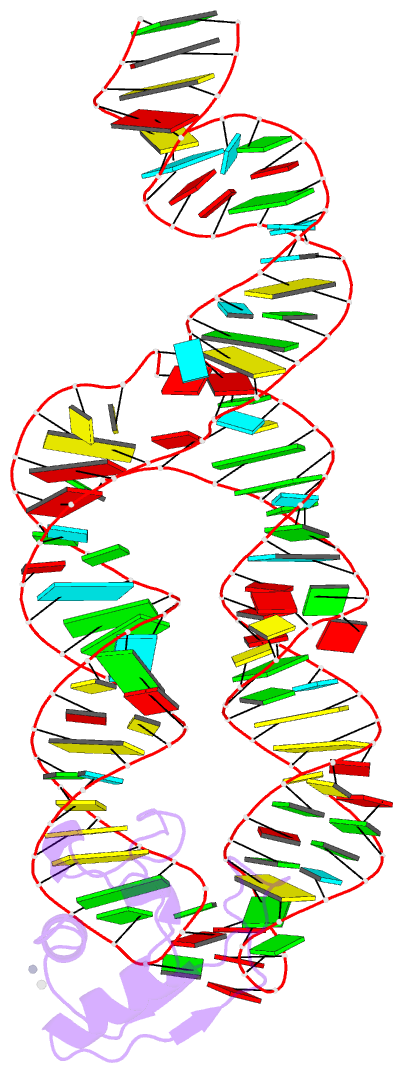
Summary information and primary citation
- PDB-id
- 1l9a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- signaling protein-RNA
- Method
- X-ray (2.9 Å)
- Summary
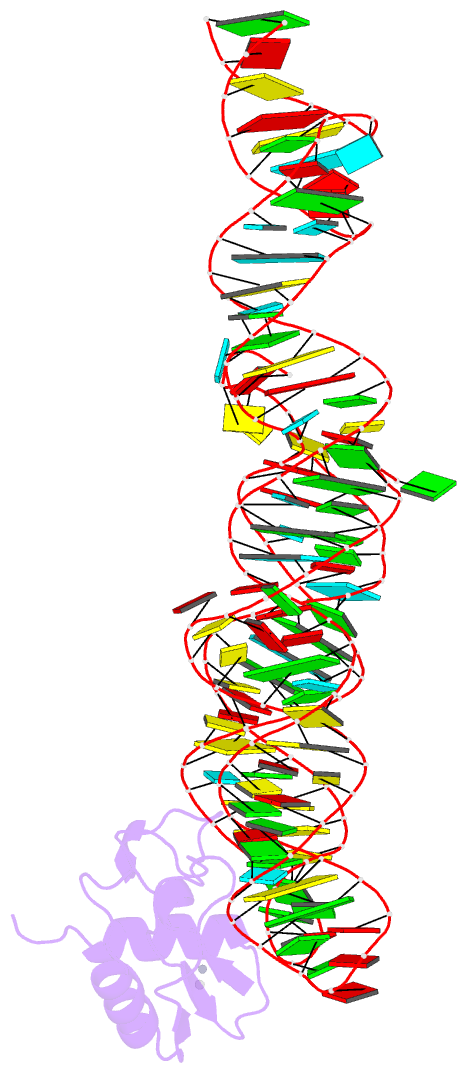
- Crystal structure of srp19 in complex with the s domain of signal recognition particle RNA
- Reference
- Oubridge C, Kuglstatter A, Jovine L, Nagai K (2002): "Crystal structure of SRP19 in complex with the S domain of SRP RNA and its implication for the assembly of the signal recognition particle." Mol.Cell, 9, 1251-1261. doi: 10.1016/S1097-2765(02)00530-0.
- Abstract
- The signal recognition particle (SRP) is a ribonucleoprotein particle involved in GTP-dependent translocation of secretory proteins across membranes. In Archaea and Eukarya, SRP19 binds to 7SL RNA and promotes the incorporation of SRP54, which contains the binding sites for GTP, the signal peptide, and the membrane-bound SRP receptor. We have determined the crystal structure of Methanococcus jannaschii SRP19 bound to the S domain of human 7SL RNA at 2.9 A resolution. SRP19 clamps the tetraloops of two branched helices (helices 6 and 8) and allows them to interact side by side. Helix 6 acts as a splint for helix 8 and partially preorganizes the binding site for SRP54 in helix 8, thereby facilitating the binding of SRP54 in assembly.