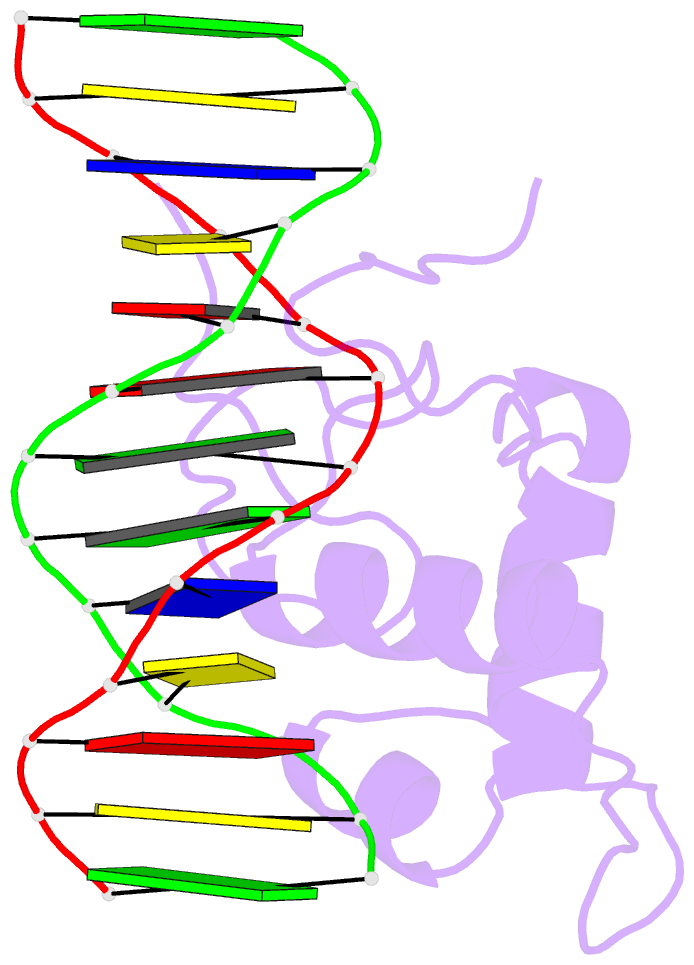
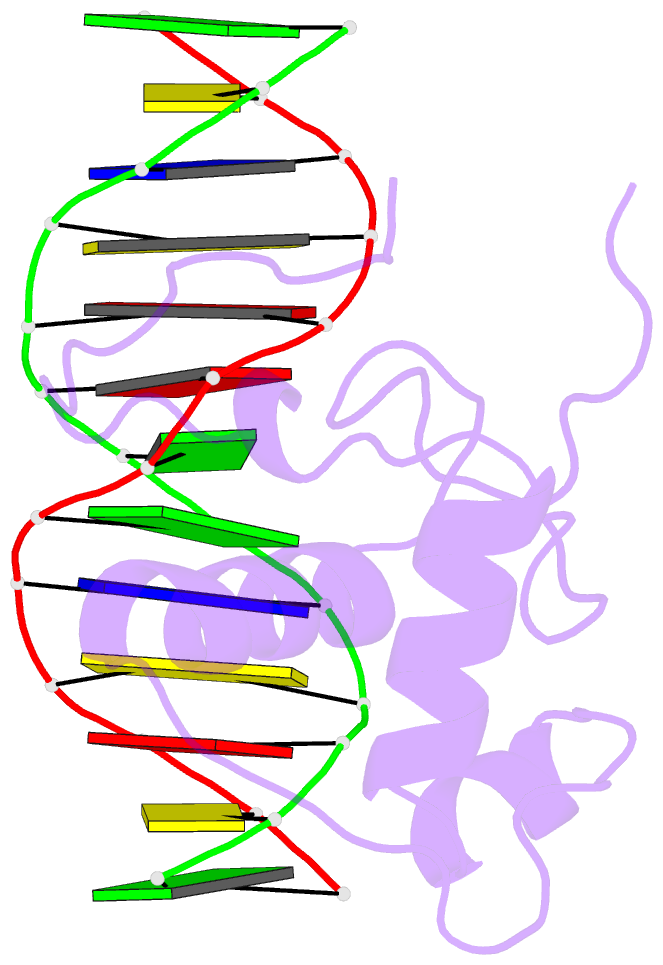
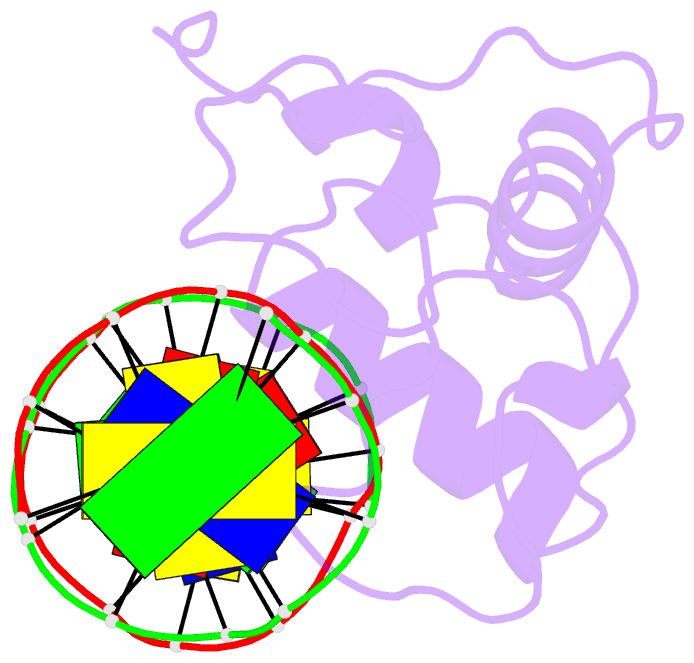
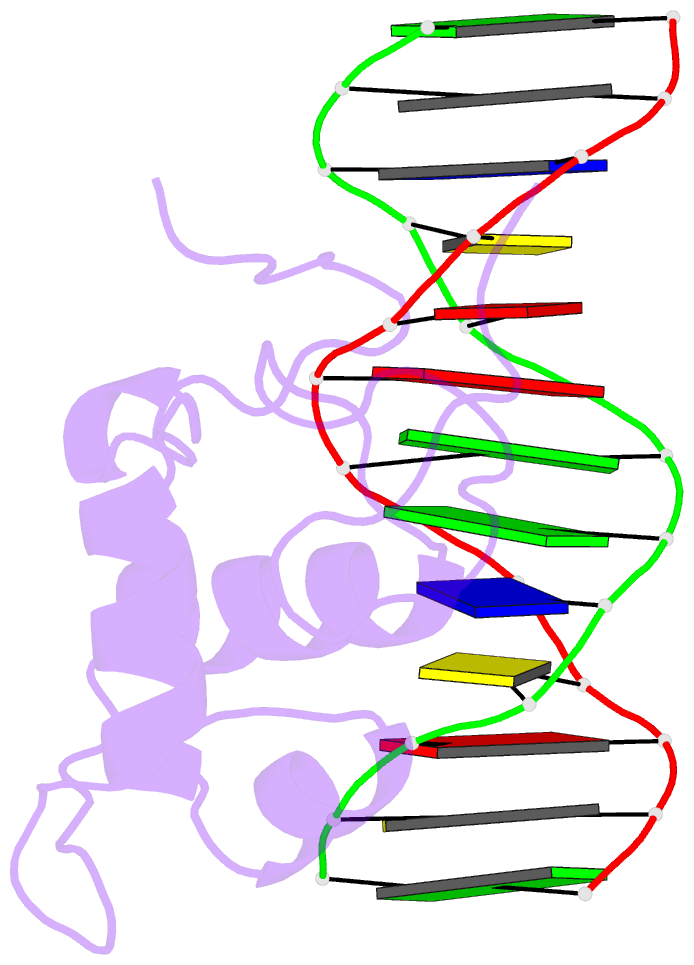
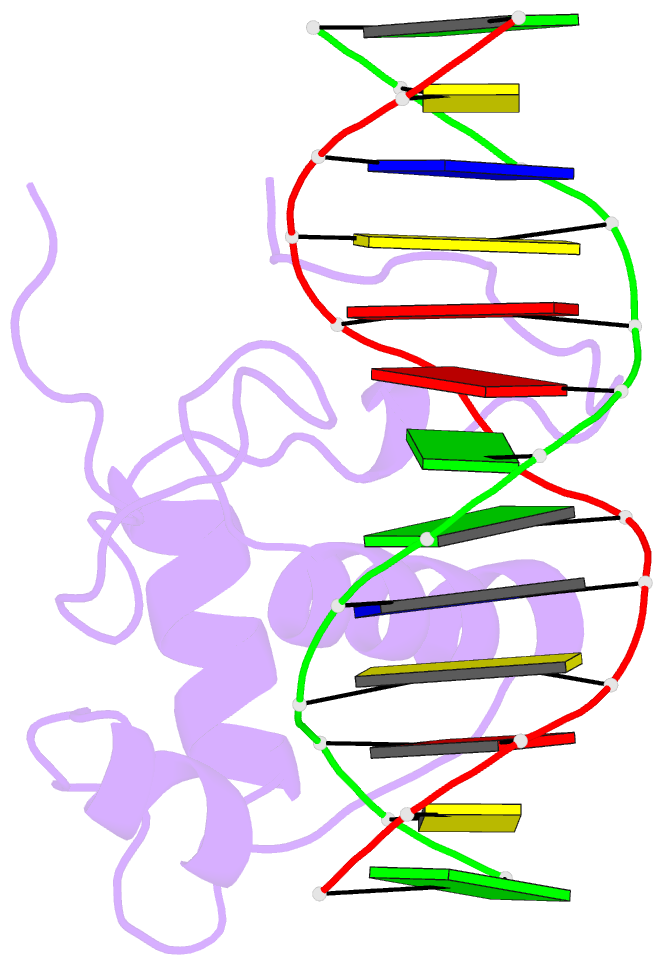
Summary information and primary citation
- PDB-id
- 1lo1; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hormone-growth factor receptor-DNA
- Method
- NMR
- Summary
- Estrogen related receptor 2 DNA binding domain in complex with DNA
- Reference
- Gearhart MD, Holmbeck SMA, Evans RM, Dyson HJ, Wright PE (2003): "Monomeric Complex of Human Orphan Estrogen Related Receptor-2 with DNA: A Pseudo-dimer Interface Mediates Extended Half-site Recognition." J.Mol.Biol., 327, 819-832. doi: 10.1016/S0022-2836(03)00183-9.
- Abstract
- While most nuclear receptors bind DNA as homo or heterodimers, the human estrogen related receptors (hERRs) are members of a subfamily of orphan receptors that bind DNA as monomers. We have determined the solution structure of the DNA binding domain (DBD) of hERR2 bound to its cognate DNA. The structure and base interactions of the core DBD are similar to those of other nuclear receptors. However, high-affinity, sequence-specific DNA binding as a monomer necessitates formation of additional base contacts outside the core DBD. This is accomplished using a modified guanosine-binding "AT-hook" within the C-terminal extension (CTE) flanking the DBD, which makes base-specific minor groove interactions. The structure of the CTE is stabilized both by interactions with the DNA and by packing against a region of the core DBD normally reserved for dimerization. This pseudo-dimer interface provides a basis for the expansion of DNA recognition and suggests a mechanism through which dimerization may have evolved from an ancestral monomeric receptor.