Summary information and primary citation
- PDB-id
- 1m07; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.8 Å)
- Summary
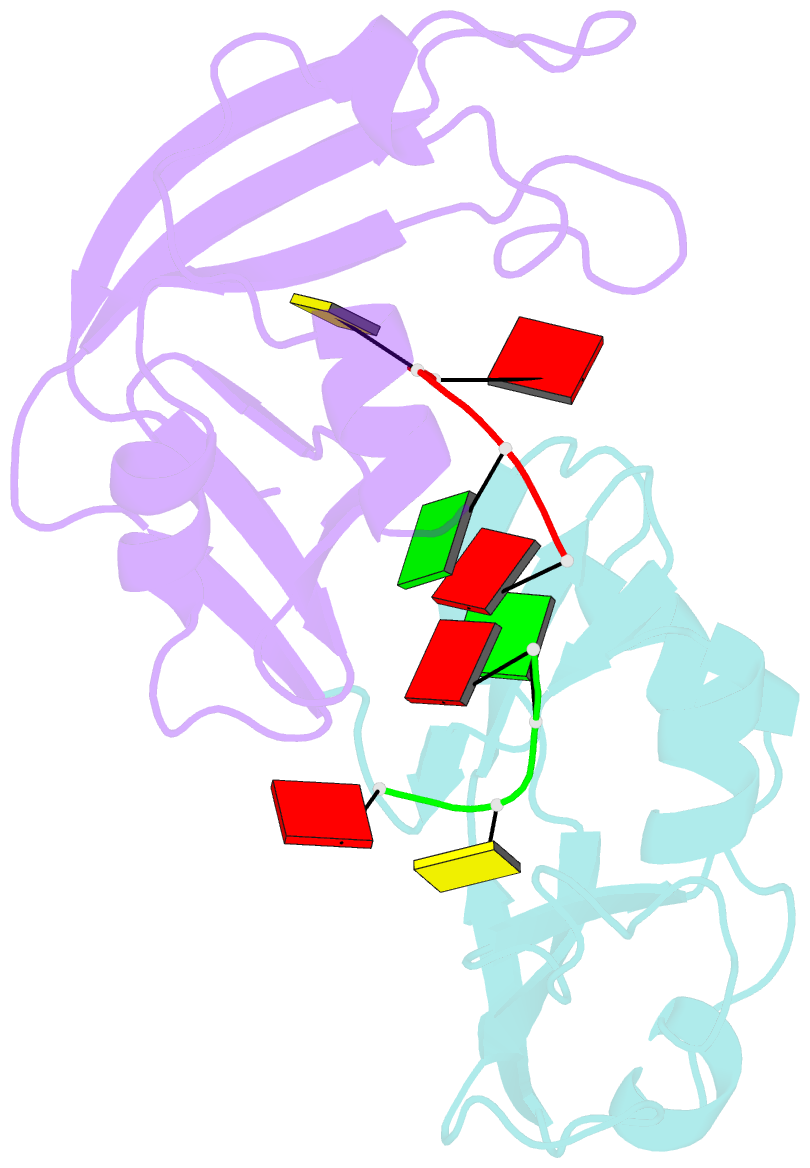
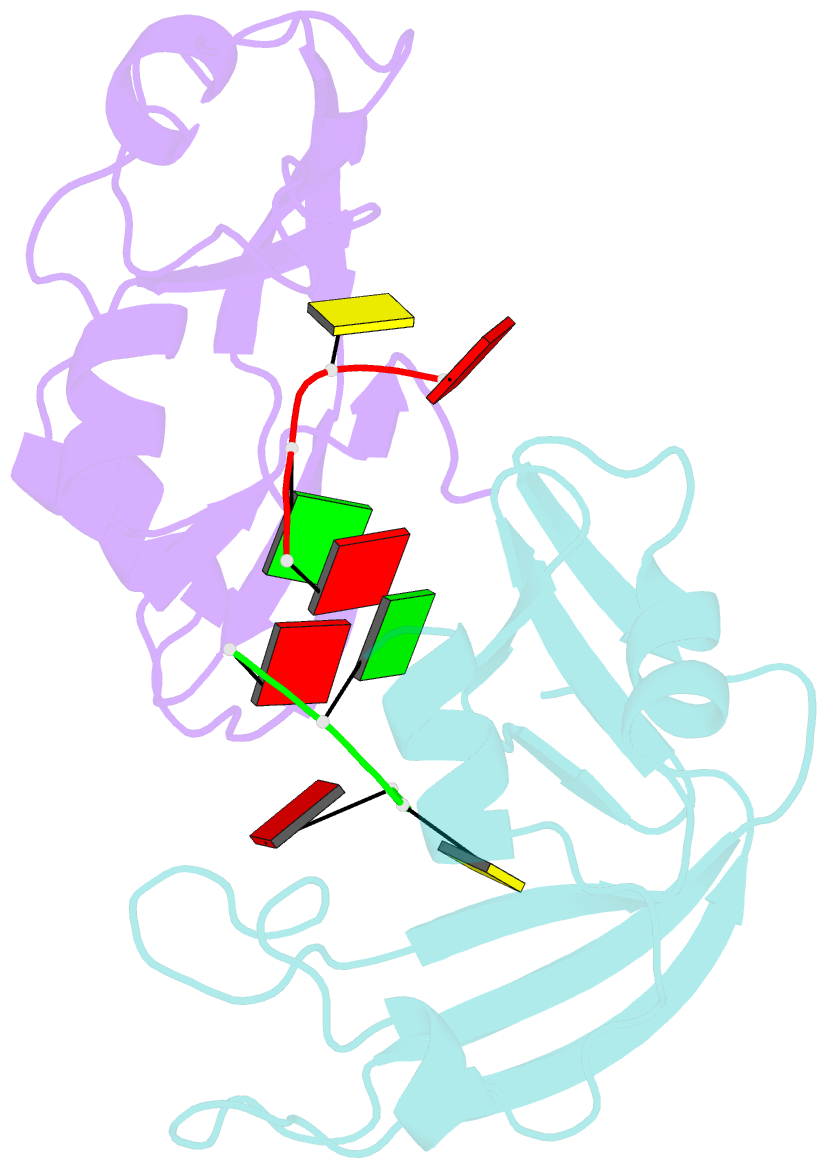
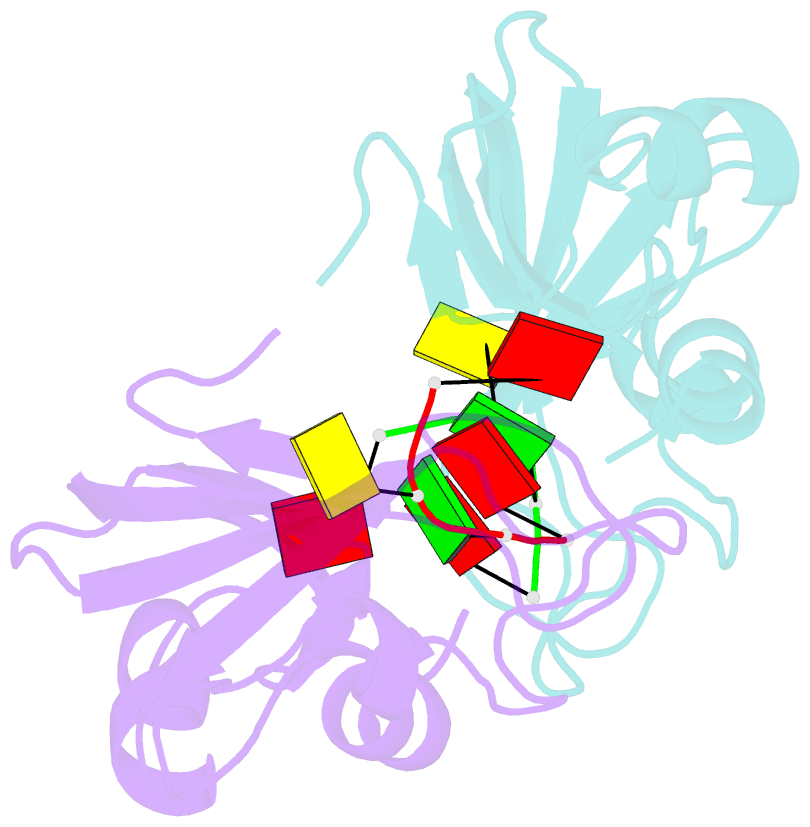
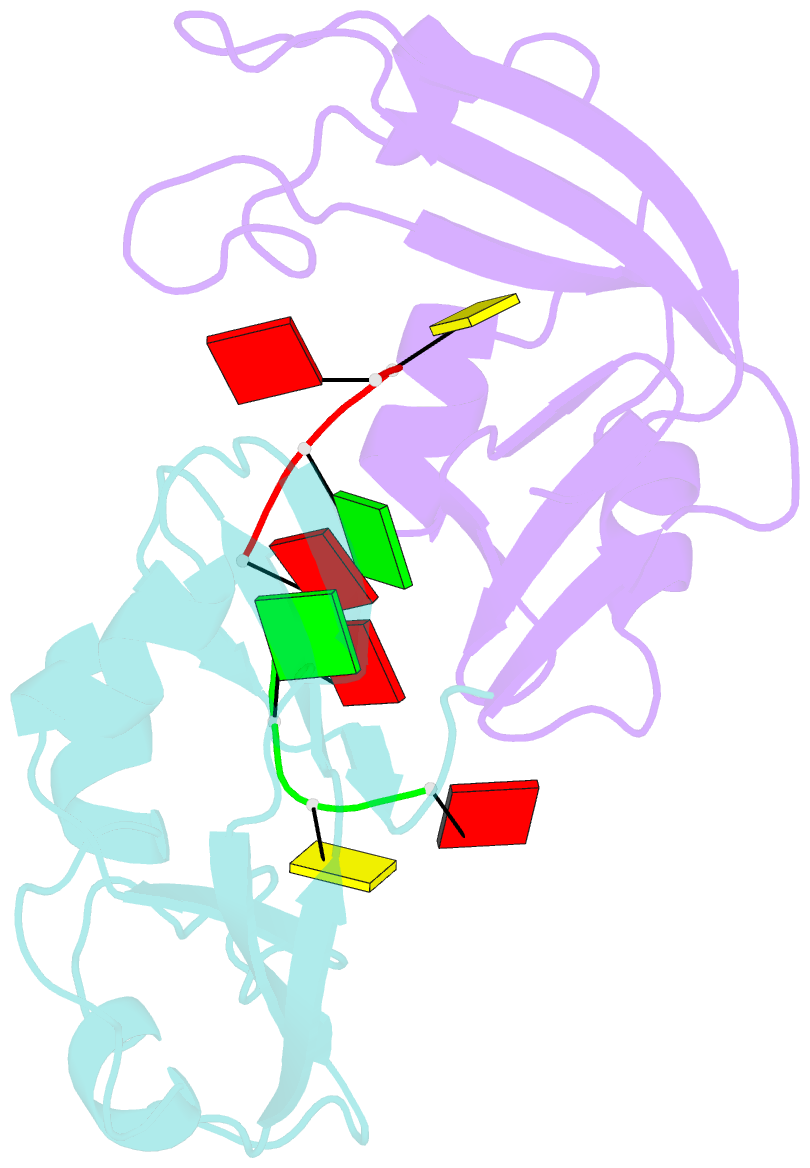
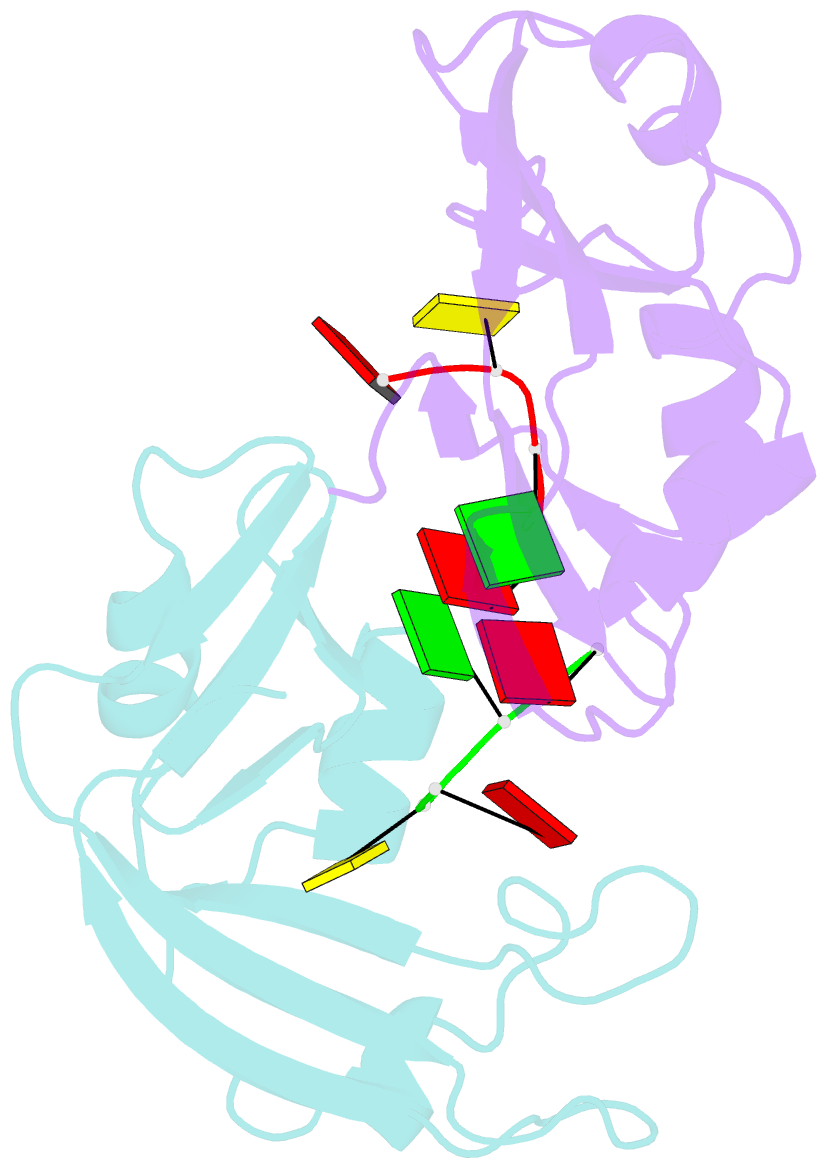
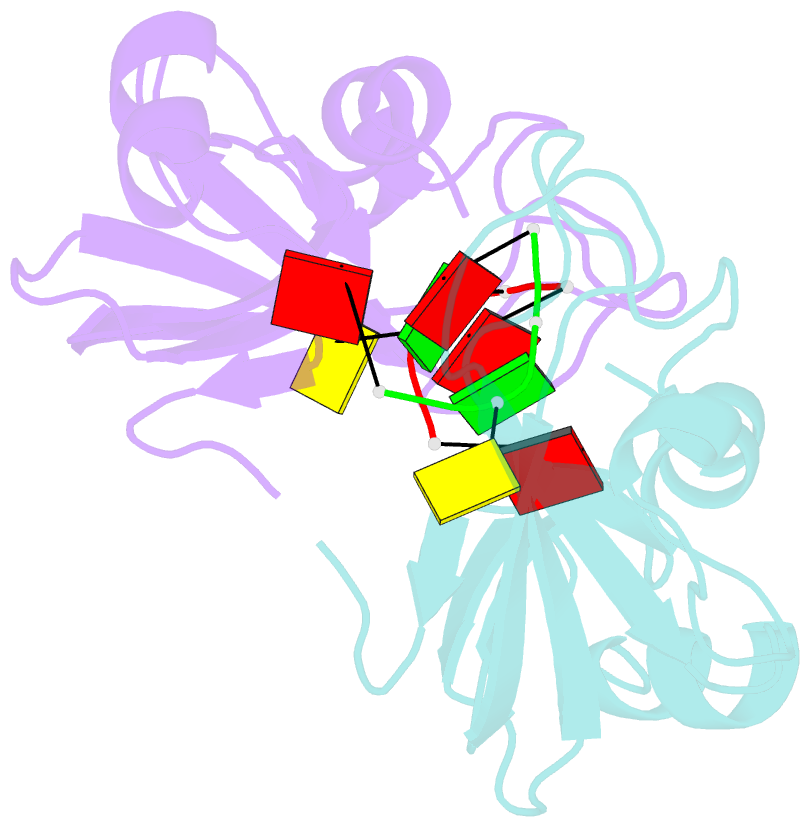
- Residues involved in the catalysis and base specificity of cytotoxic ribonuclease from bullfrog (rana catesbeiana)
- Reference
- Leu Y-J, Chern S-S, Wang S-C, Hsiao Y-Y, Amiraslanov I, Liaw Y-C, Liao Y-D (2003): "Residues involved in the catalysis, base specificity, and cytotoxicity of ribonuclease from Rana catesbeiana based upon mutagenesis and X-ray crystallography." J.Biol.Chem., 278, 7300-7309. doi: 10.1074/jbc.M206701200.
- Abstract
- The Rana catesbeiana (bullfrog) ribonucleases, which belong to the RNase A superfamily, exert cytotoxicity toward tumor cells. RC-RNase, the most active among frog ribonucleases, has a unique base preference for pyrimidine-guanine rather than pyrimidine-adenine in RNase A. Residues of RC-RNase involved in base specificity and catalytic activity were determined by site-directed mutagenesis, k(cat)/K(m) analysis toward dinucleotides, and cleavage site analysis of RNA substrate. The results show that Pyr-1 (N-terminal pyroglutamate), Lys-9, and Asn-38 along with His-10, Lys-35, and His-103 are involved in catalytic activity, whereas Pyr-1, Thr-39, Thr-70, Lys-95, and Glu-97 are involved in base specificity. The cytotoxicity of RC-RNase is correlated, but not proportional to, its catalytic activity. The crystal structure of the RC-RNase.d(ACGA) complex was determined at 1.80 A resolution. Residues Lys-9, His-10, Lys-35, and His-103 interacted directly with catalytic phosphate at the P(1) site, and Lys-9 was stabilized by hydrogen bonds contributed by Pyr-1, Tyr-28, and Asn-38. Thr-70 acts as a hydrogen bond donor for cytosine through Thr-39 and determines B(1) base specificity. Interestingly, Pyr-1 along with Lys-95 and Glu-97 form four hydrogen bonds with guanine at B(2) site and determine B(2) base specificity.