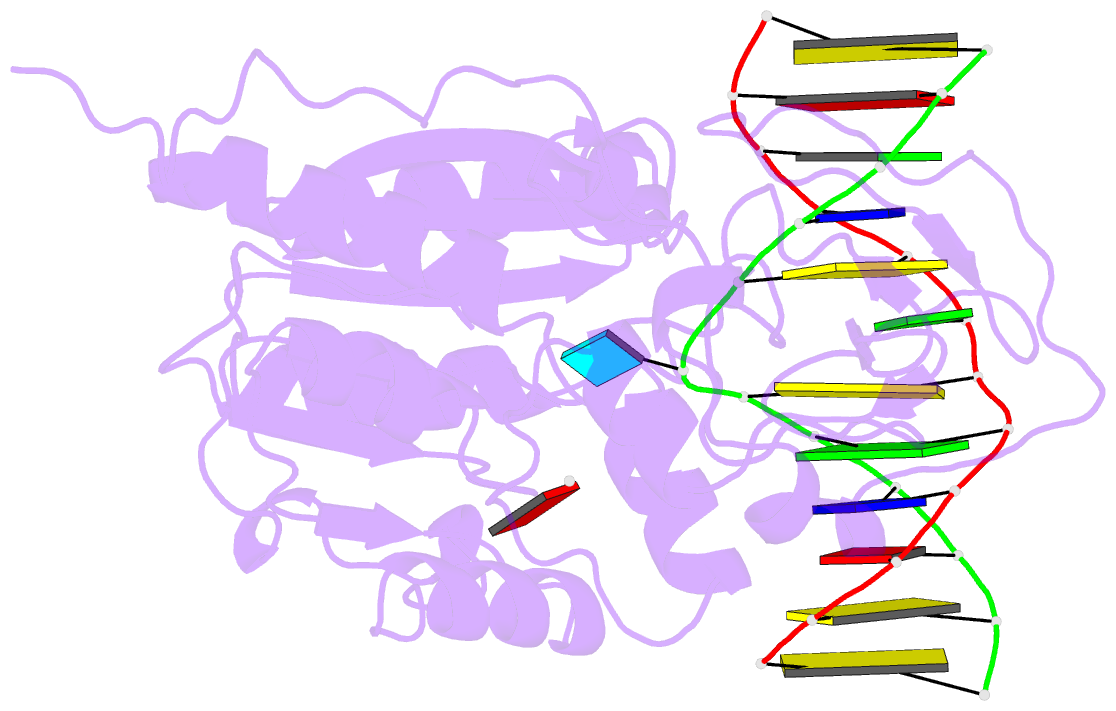
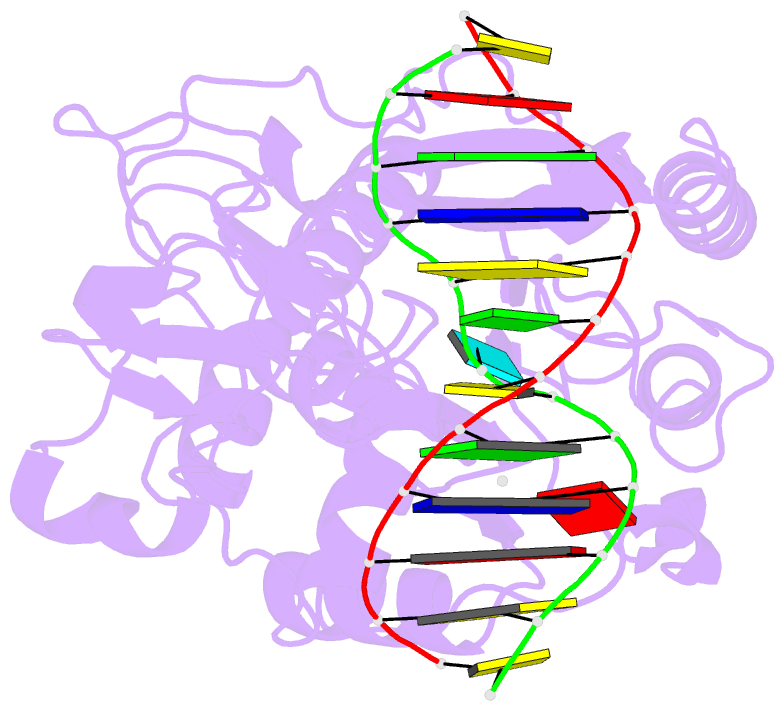
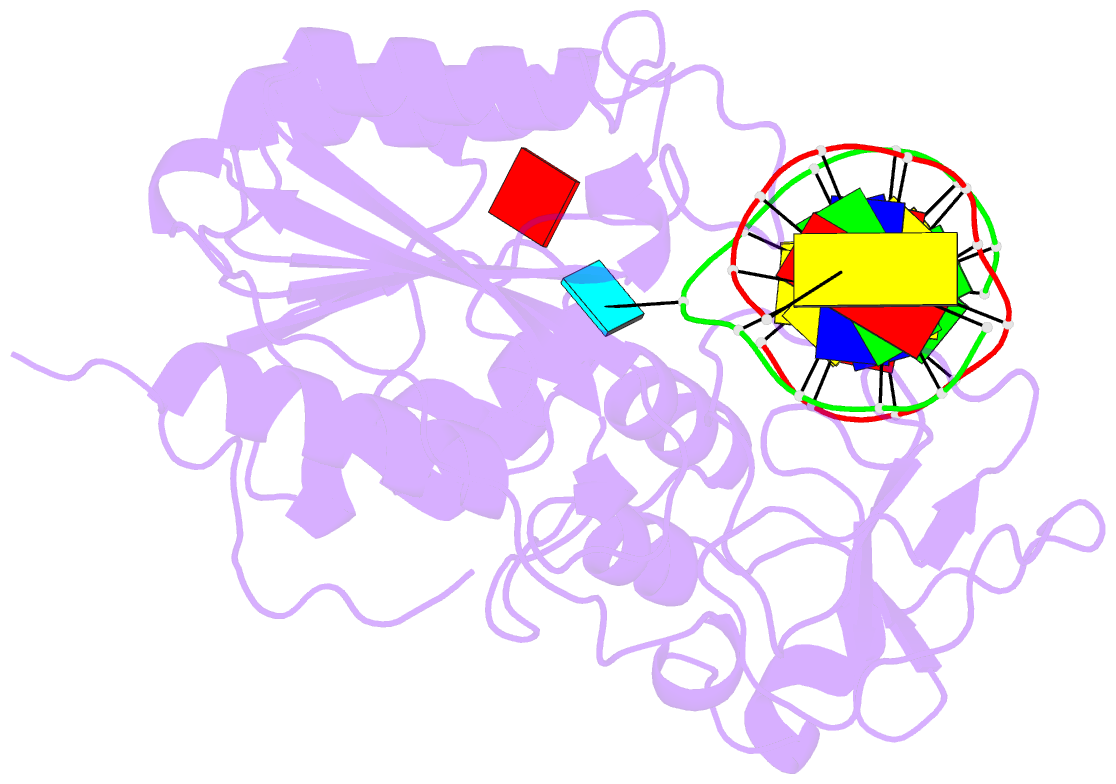
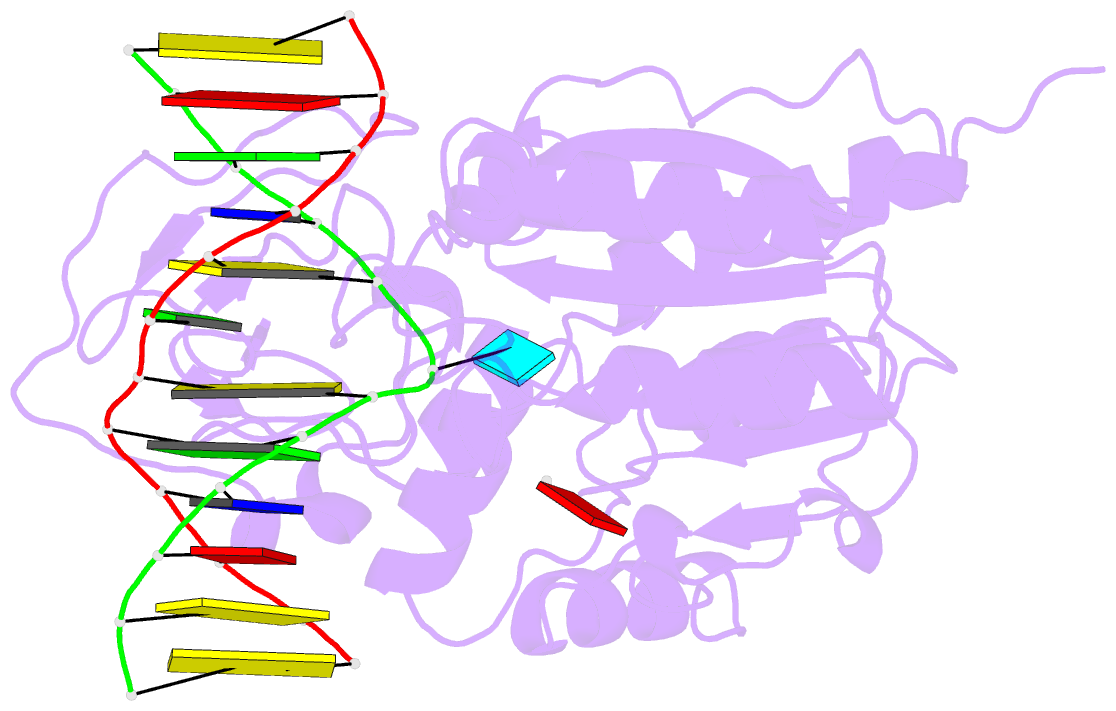
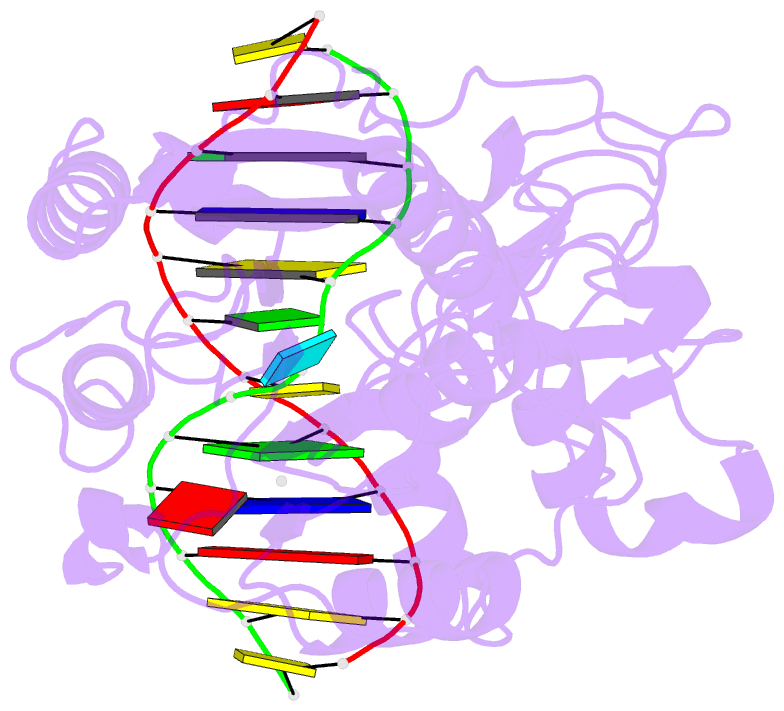
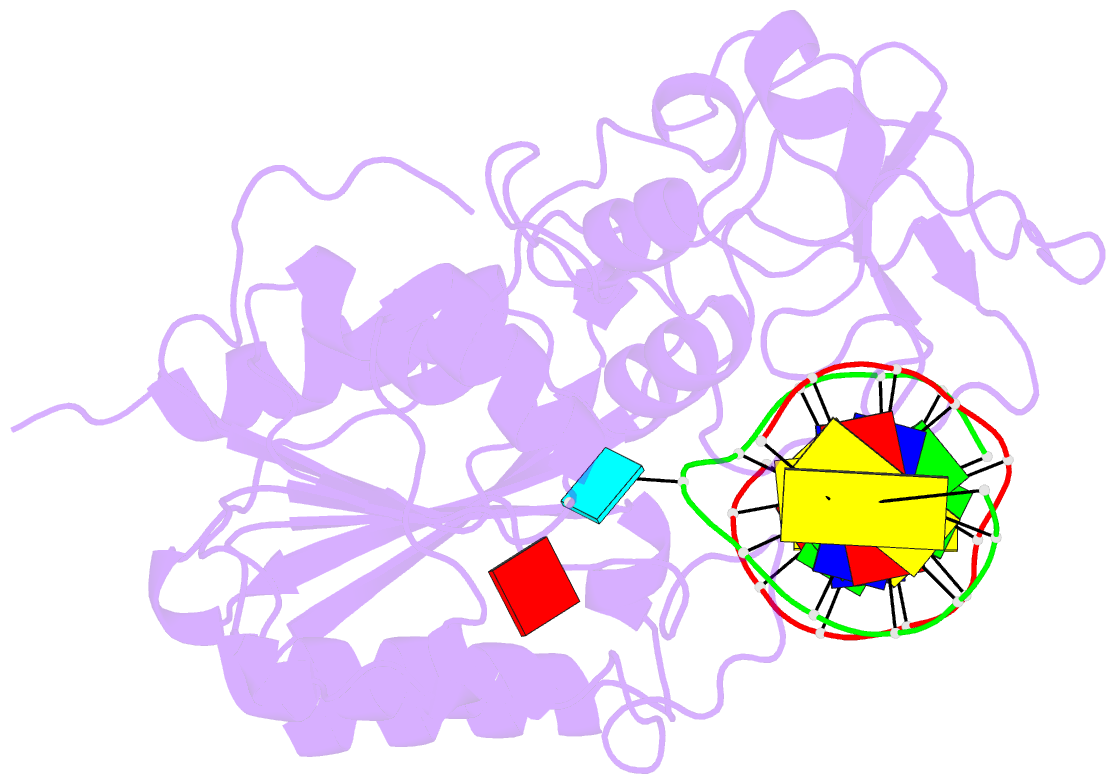
Summary information and primary citation
- PDB-id
- 1m0e; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.5 Å)
- Summary
- Zebularine: a novel DNA methylation inhibitor that forms a covalent complex with DNA methyltransferase
- Reference
- Zhou L, Cheng X, Connolly BA, Dickman MJ, Hurd PJ, Hornby DP (2002): "ZEBULARINE: A NOVEL DNA METHYLATION INHIBITOR THAT FORMS A COVALENT COMPLEX WITH DNA METHYLTRANSFERASES." J.MOL.BIOL., 321, 591-599. doi: 10.1016/S0022-2836(02)00676-9.
- Abstract
- Mechanism-based inhibitors of enzymes, which mimic reactive intermediates in the reaction pathway, have been deployed extensively in the analysis of metabolic pathways and as candidate drugs. The inhibition of cytosine-[C5]-specific DNA methyltransferases (C5 MTases) by oligodeoxynucleotides containing 5-azadeoxycytidine (AzadC) and 5-fluorodeoxycytidine (FdC) provides a well-documented example of mechanism-based inhibition of enzymes central to nucleic acid metabolism. Here, we describe the interaction between the C5 MTase from Haemophilus haemolyticus (M.HhaI) and an oligodeoxynucleotide duplex containing 2-H pyrimidinone, an analogue often referred to as zebularine and known to give rise to high-affinity complexes with MTases. X-ray crystallography has demonstrated the formation of a covalent bond between M.HhaI and the 2-H pyrimidinone-containing oligodeoxynucleotide. This observation enables a comparison between the mechanisms of action of 2-H pyrimidinone with other mechanism-based inhibitors such as FdC. This novel complex provides a molecular explanation for the mechanism of action of the anti-cancer drug zebularine.