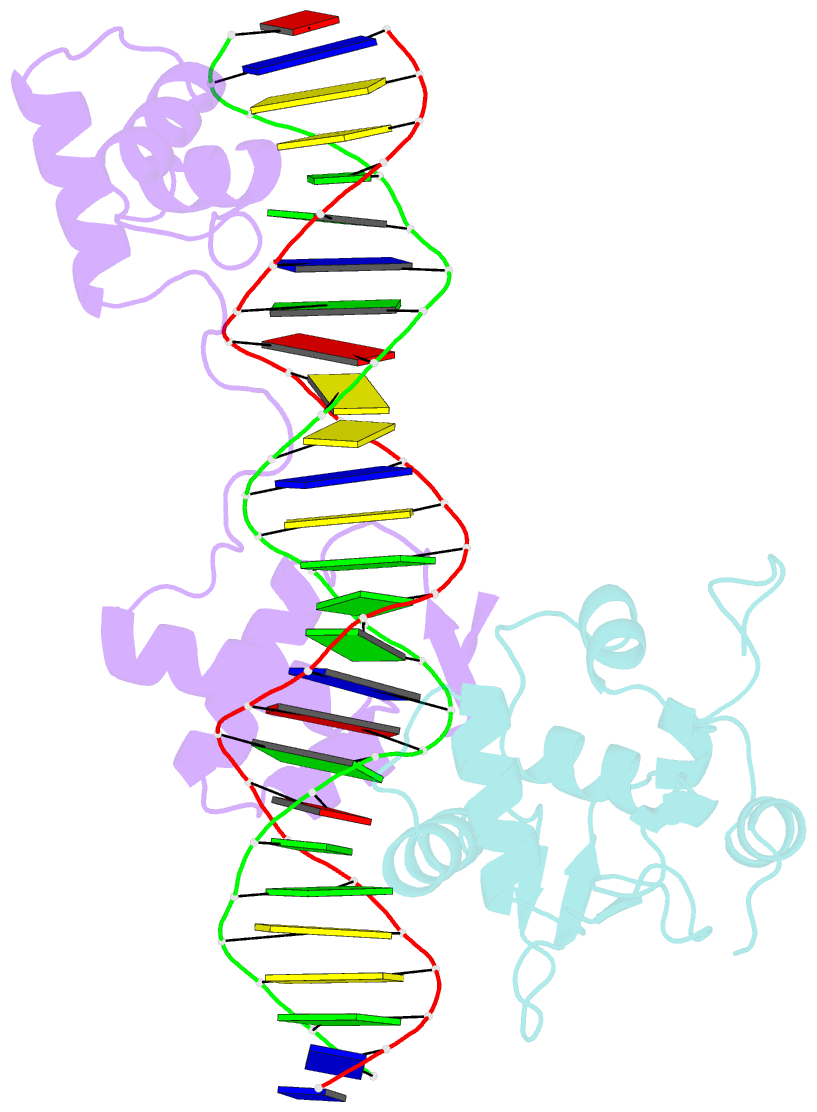
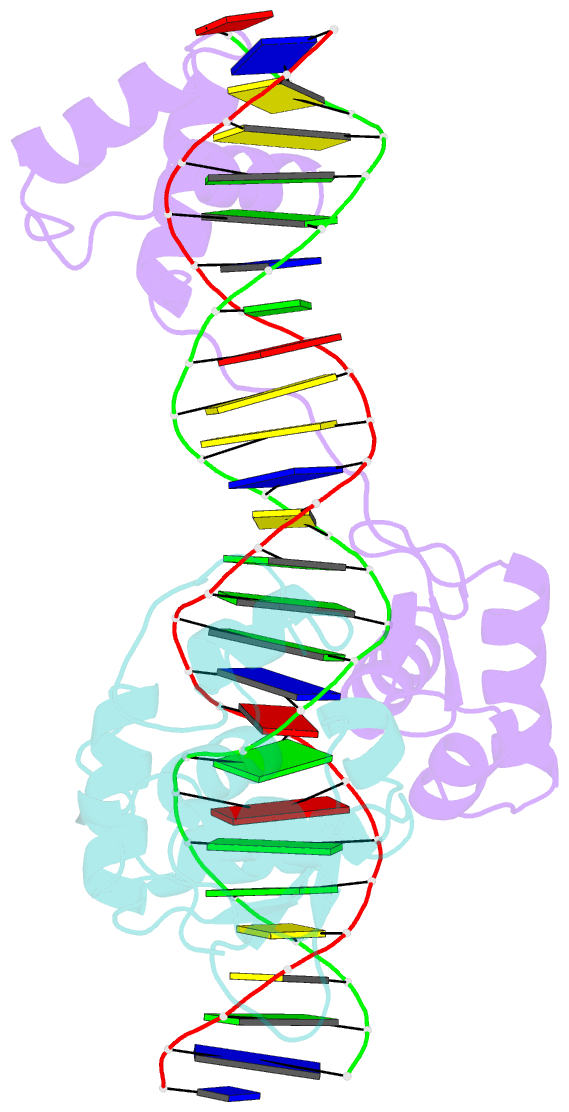
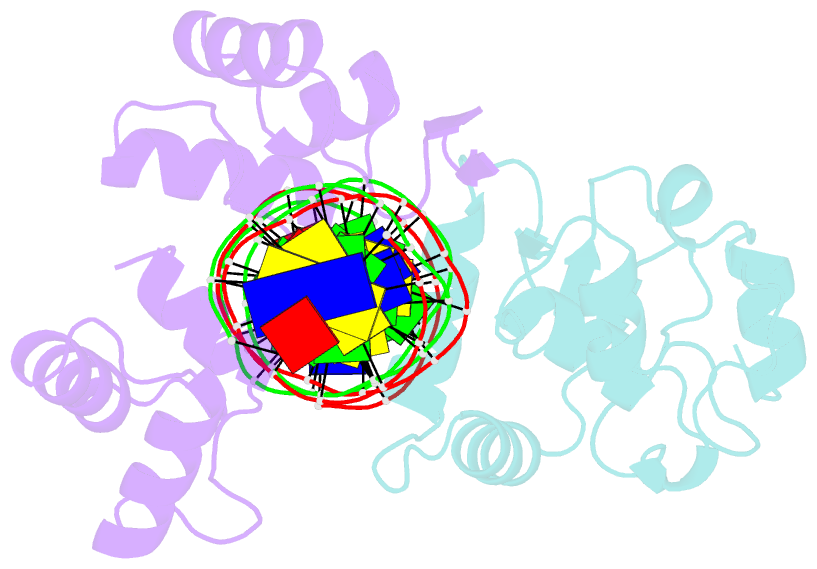
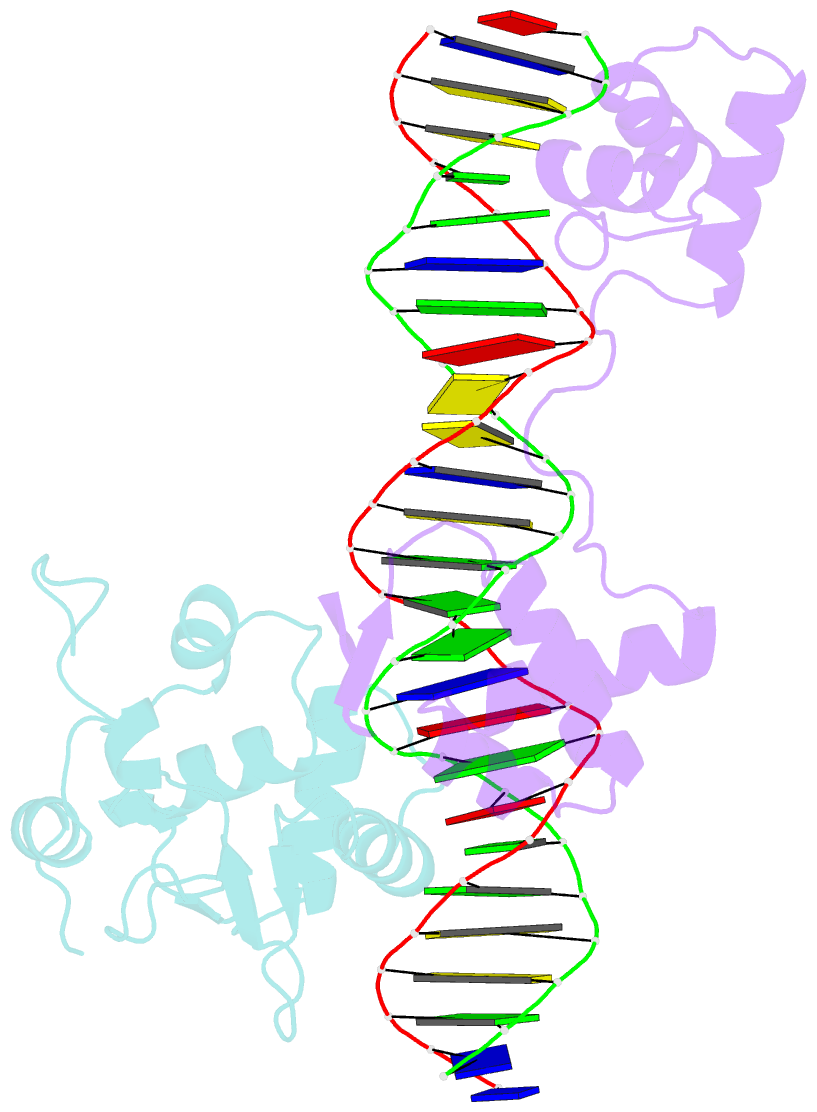
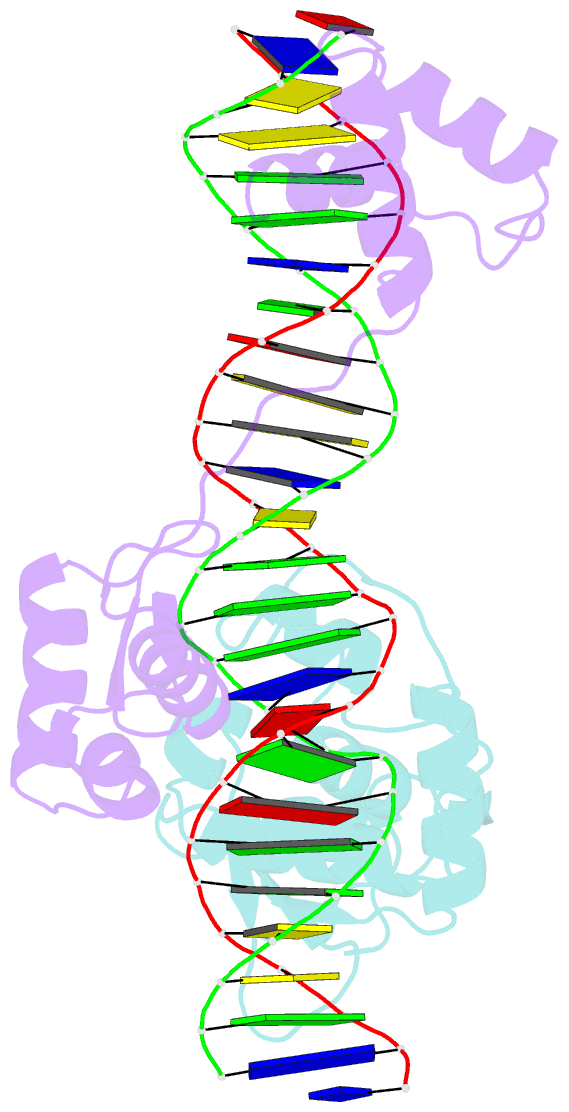
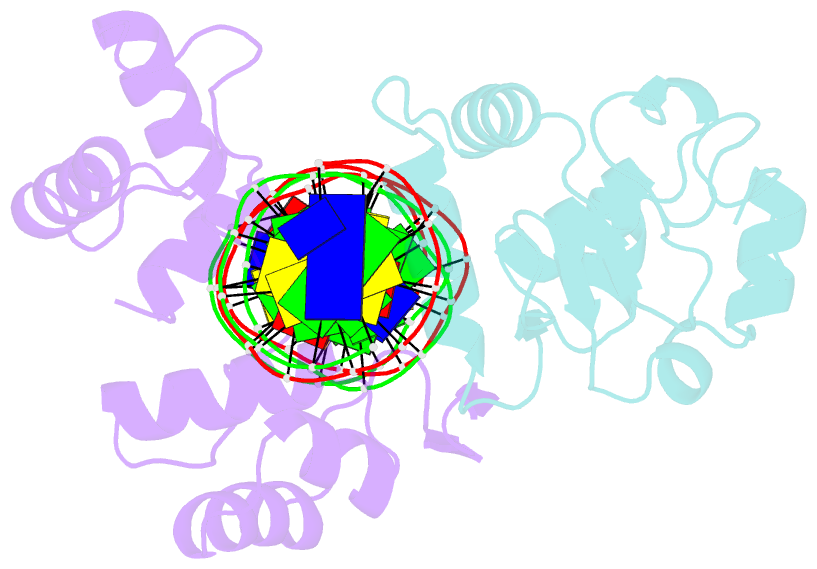
Summary information and primary citation
- PDB-id
- 1mdm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.8 Å)
- Summary
- Inhibited fragment of ets-1 and paired domain of pax5 bound to DNA
- Reference
- Garvie CW, Pufall MA, Graves BJ, Wolberger C (2002): "STRUCTURAL ANALYSIS OF THE AUTOINHIBITION OF ETS-1 AND ITS ROLE IN PROTEIN PARTNERSHIPS." J.Biol.Chem., 277, 45529-45536. doi: 10.1074/jbc.M206327200.
- Abstract
- The DNA-binding activity of the eukaryotic transcription factor Ets-1 (E26 avian erythroblastosis virus oncogene-E twenty-six) is negatively regulated by inhibitory regions that flank the ETS domain. Based on the results of solution studies, these N- and C-terminal inhibitory regions have been proposed to pack against the ETS domain and form an autoinhibitory module whose N terminus partially unfolds upon binding of Ets-1 to DNA. Mutations that disrupt autoinhibition of DNA binding also cause a structural change in the inhibitory region. We report here a crystallographic study of fragments of Ets-1 that provide structural details of the inhibitory module and the structural transition that accompanies DNA binding. The structures of free and DNA-bound Ets-1 fragments containing the ETS domain and the inhibitory regions confirm that the N-terminal inhibitory region contains two alpha-helices one of which unfolds upon Ets-1 binding to DNA. The observations from the crystal structure, coupled with mutagenesis experiments, allow us to propose a model for the inhibited form of Ets-1 and lend insight into the flexible interaction between Ets-1 and the acute myeloid leukemia 1 protein, AML1 (RUNX1).