Summary information and primary citation
- PDB-id
- 1mje; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- gene regulation-antitumor protein-DNA
- Method
- X-ray (3.5 Å)
- Summary
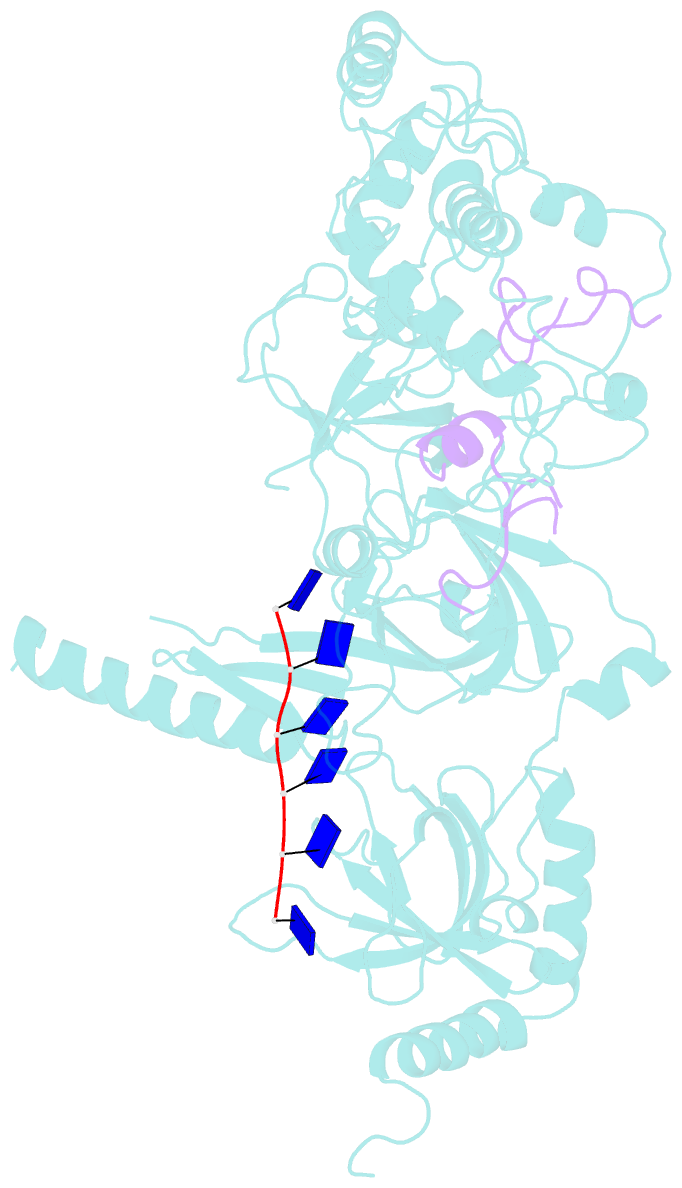
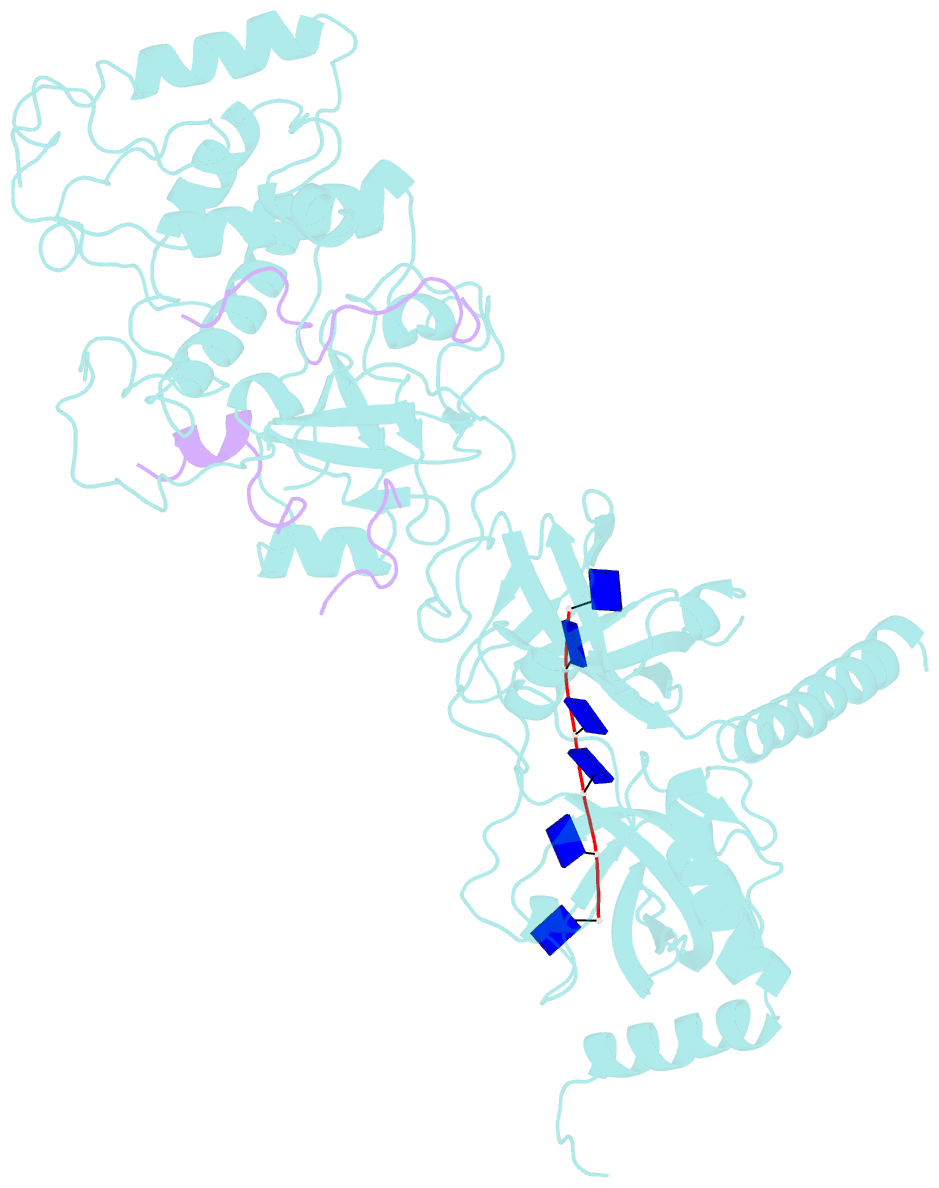
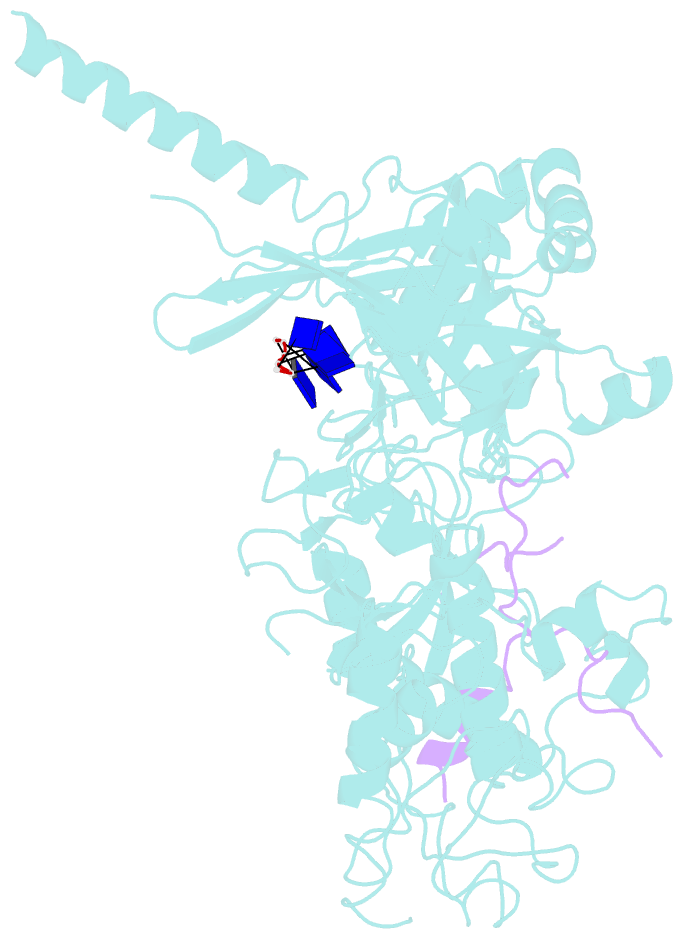
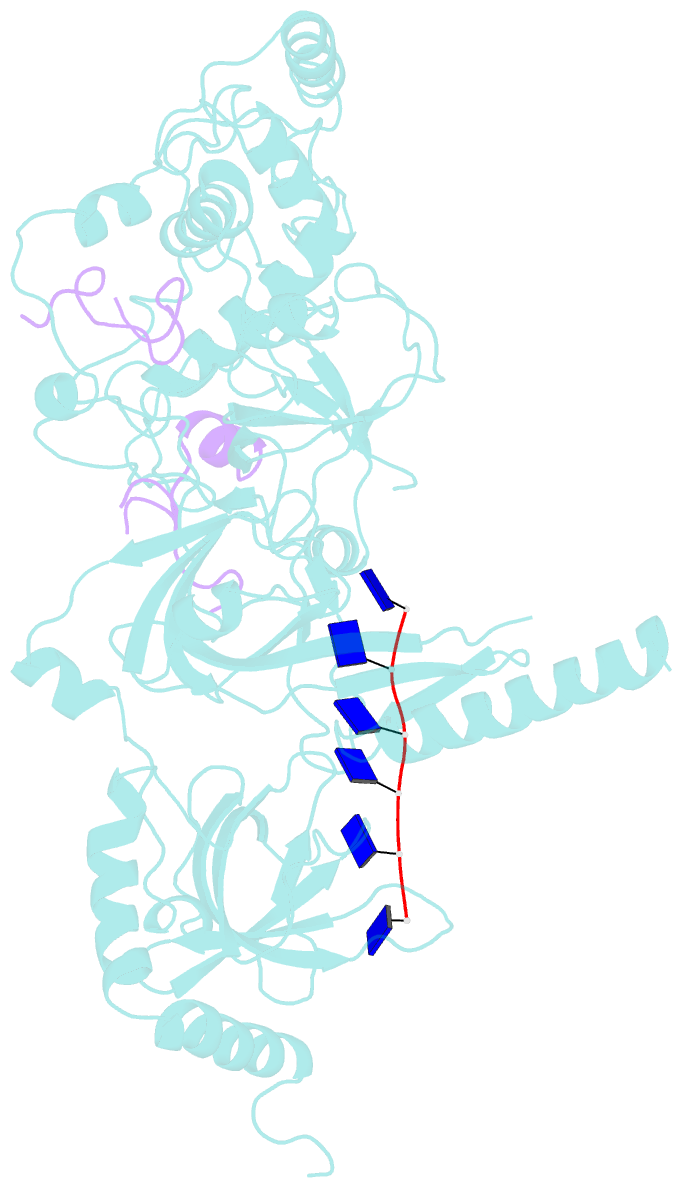
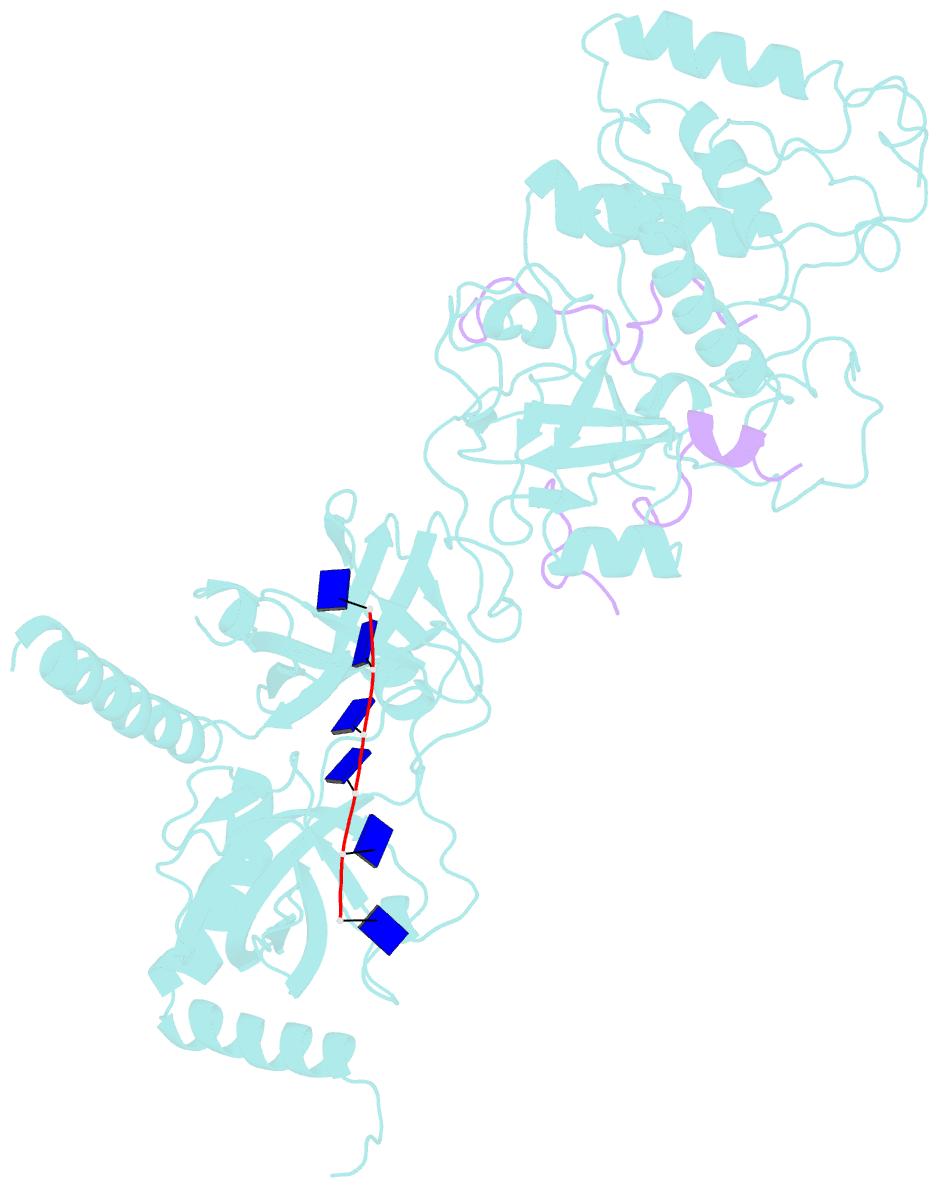
- Structure of a brca2-dss1-ssDNA complex
- Reference
- Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP (2002): "BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure." Science, 297, 1837-1848. doi: 10.1126/science.297.5588.1837.
- Abstract
- Mutations in the BRCA2 (breast cancer susceptibility gene 2) tumor suppressor lead to chromosomal instability due to defects in the repair of double-strand DNA breaks (DSBs) by homologous recombination, but BRCA2's role in this process has been unclear. Here, we present the 3.1 angstrom crystal structure of a approximately 90-kilodalton BRCA2 domain bound to DSS1, which reveals three oligonucleotide-binding (OB) folds and a helix-turn-helix (HTH) motif. We also (i) demonstrate that this BRCA2 domain binds single-stranded DNA, (ii) present its 3.5 angstrom structure bound to oligo(dT)9, (iii) provide data that implicate the HTH motif in dsDNA binding, and (iv) show that BRCA2 stimulates RAD51-mediated recombination in vitro. These findings establish that BRCA2 functions directly in homologous recombination and provide a structural and biochemical basis for understanding the loss of recombination-mediated DSB repair in BRCA2-associated cancers.