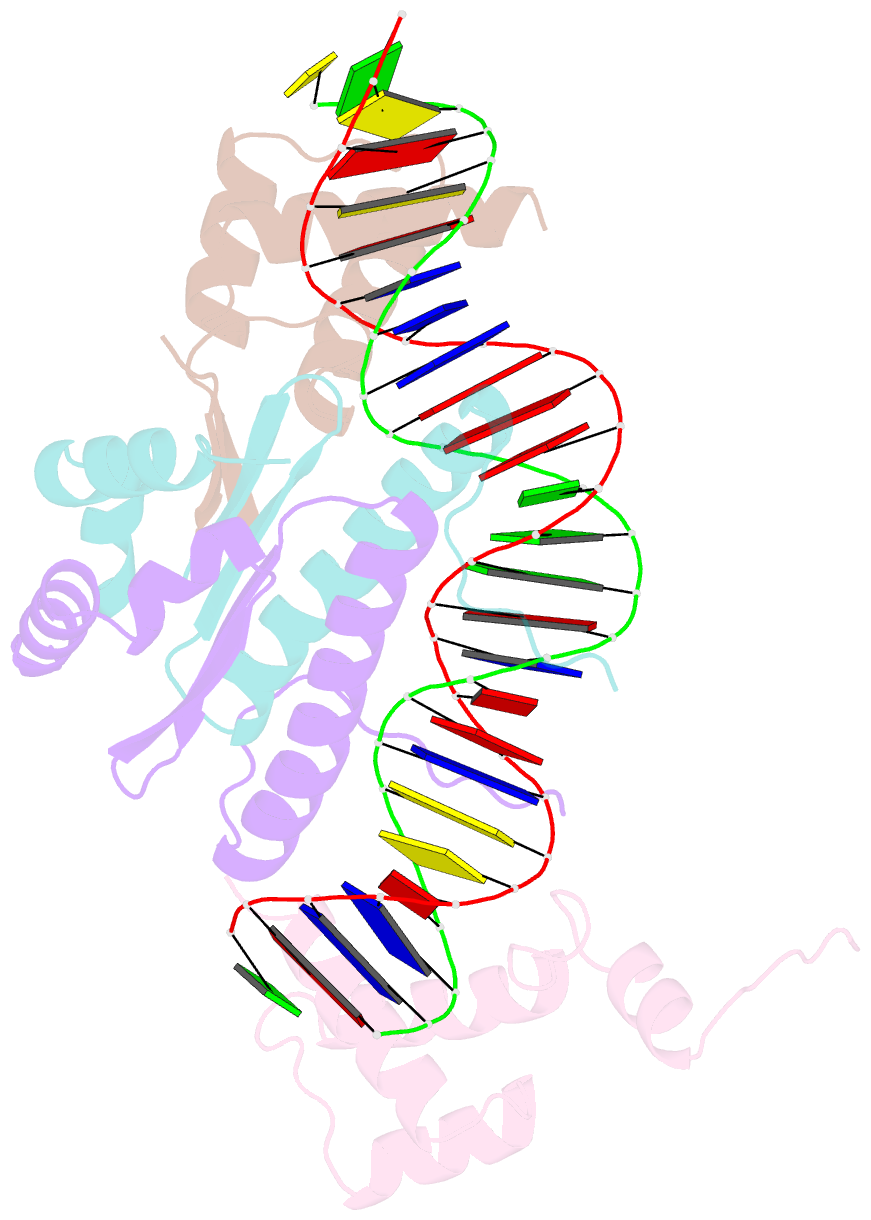
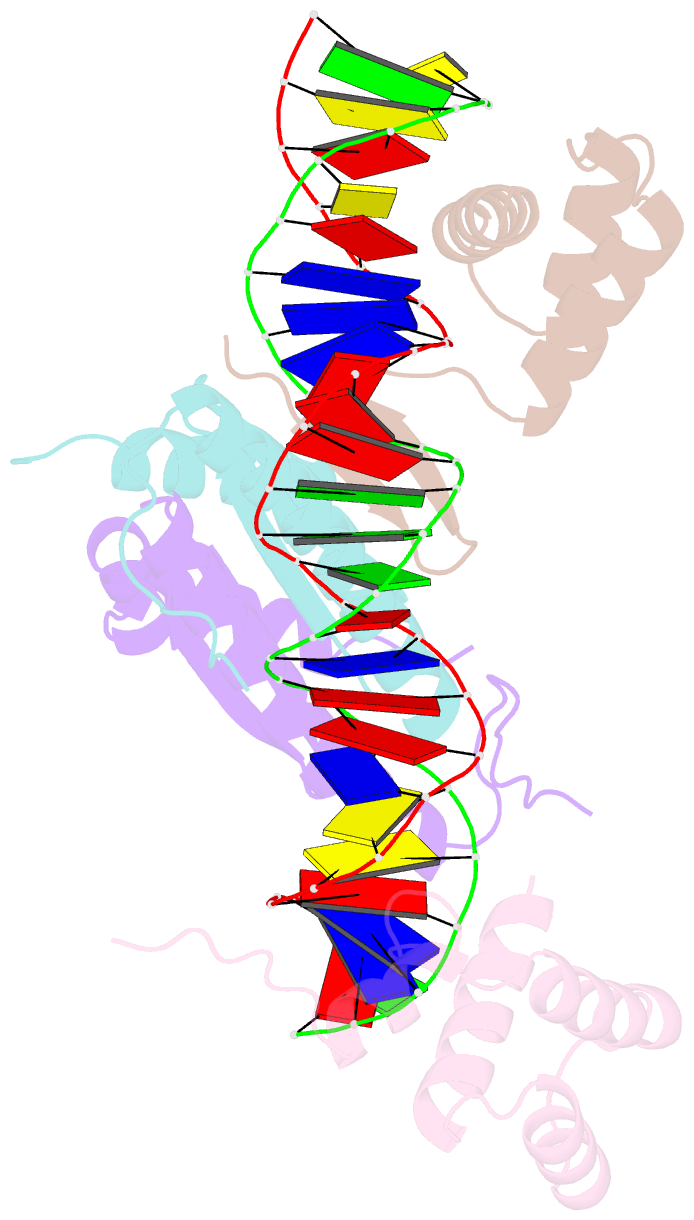
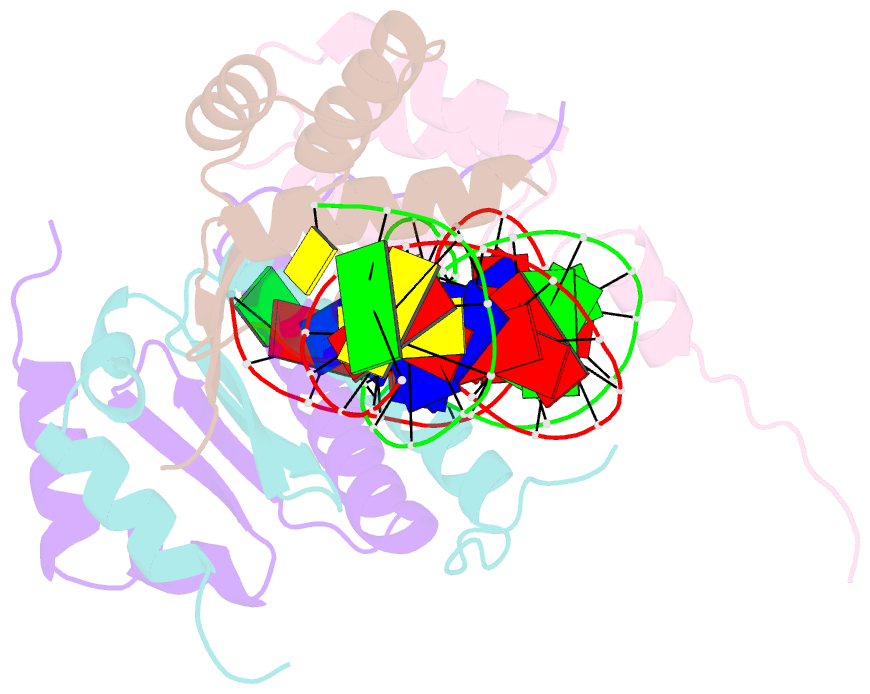
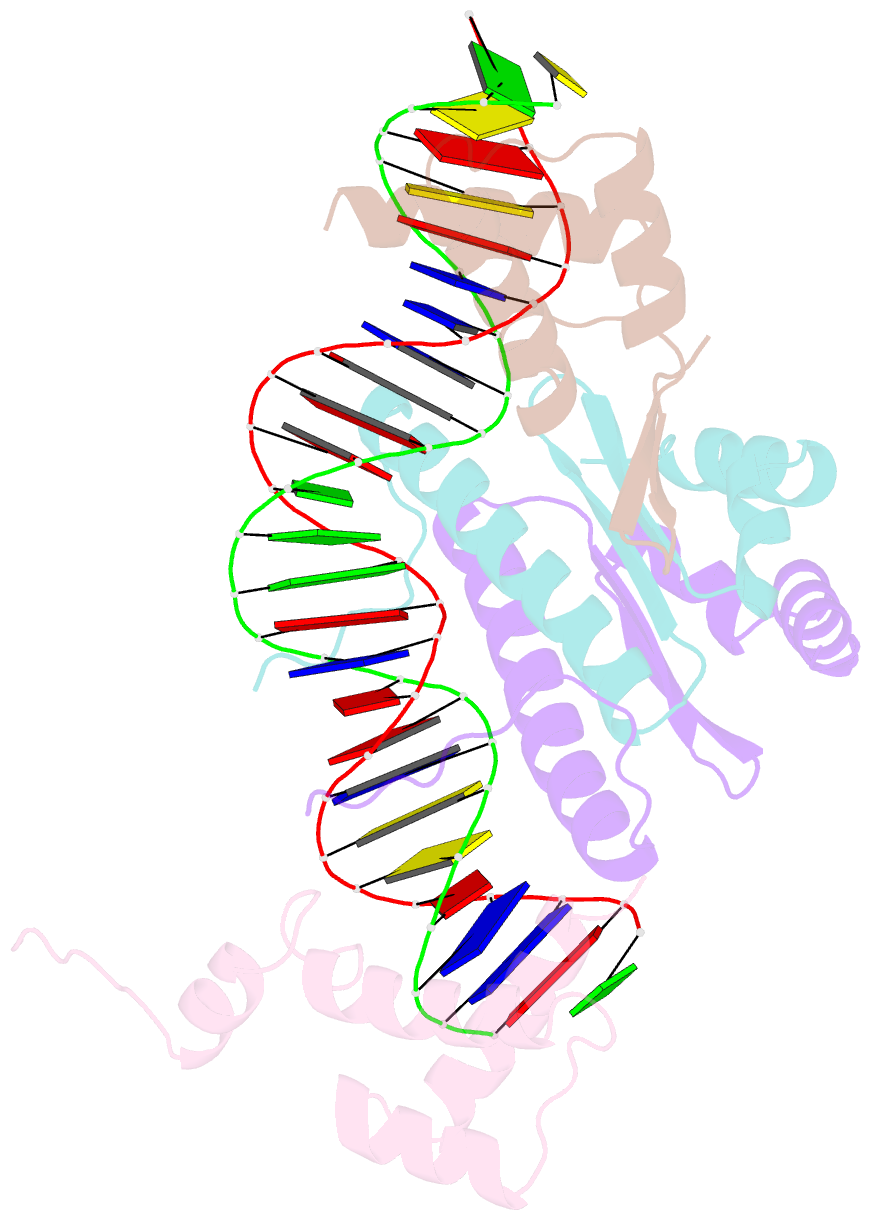
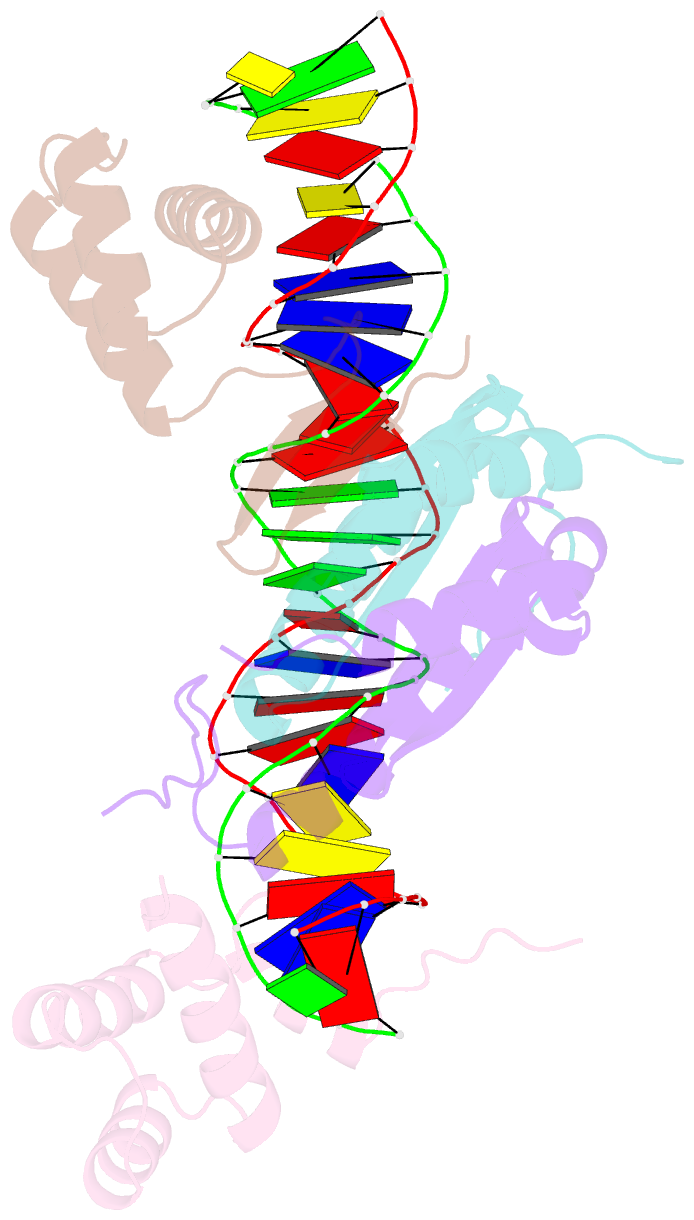
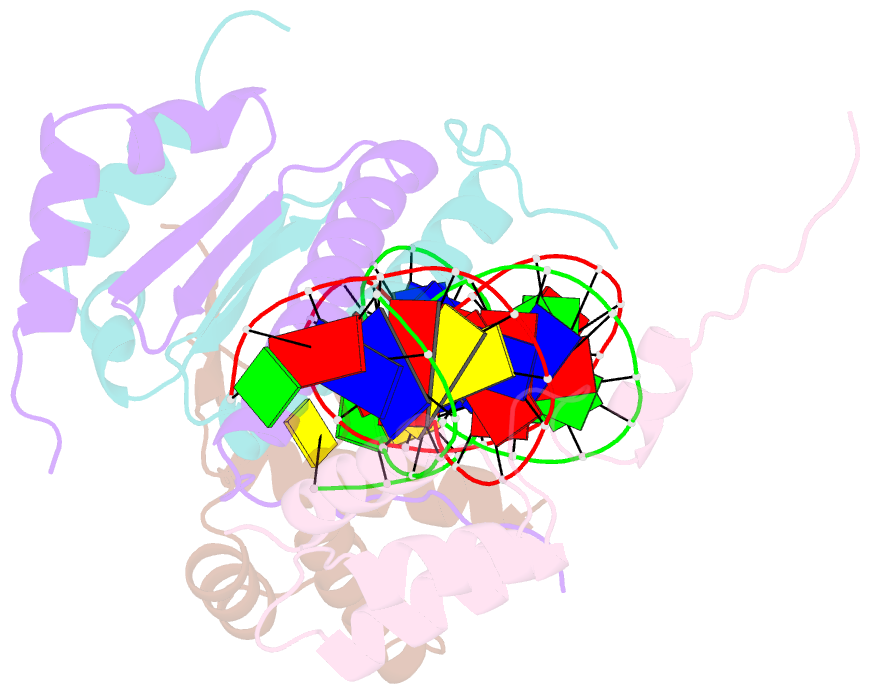
Summary information and primary citation
- PDB-id
- 1mnm; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.25 Å)
- Summary
- Yeast matalpha2-mcm1-DNA ternary transcription complex crystal structure
- Reference
- Tan S, Richmond TJ (1998): "Crystal structure of the yeast MATalpha2/MCM1/DNA ternary complex." Nature, 391, 660-666. doi: 10.1038/35563.
- Abstract
- The structure of a complex containing the homeodomain repressor protein MATalpha2 and the MADS-box transcription factor MCM1 bound to DNA has been determined by X-ray crystallography at 2.25 A resolution. It reveals the protein-protein interactions responsible for cooperative binding of MATalpha2 and MCM1 to DNA. The otherwise flexible amino-terminal extension of the MATalpha2 homeodomain forms a beta-hairpin that grips the MCM1 surface through parallel beta-strand hydrogen bonds and close-packed, predominantly hydrophobic, side chains. DNA bending induced by MCM1 brings the two proteins closer together, facilitating their interaction. An unusual feature of the complex is that an eight-amino-acid sequence adopts an alpha-helical conformation in one of two copies of the MATalpha2 monomer and a beta-strand conformation in the other. This 'chameleon' sequence of MATalpha2 may be important for recognizing natural operator sites.