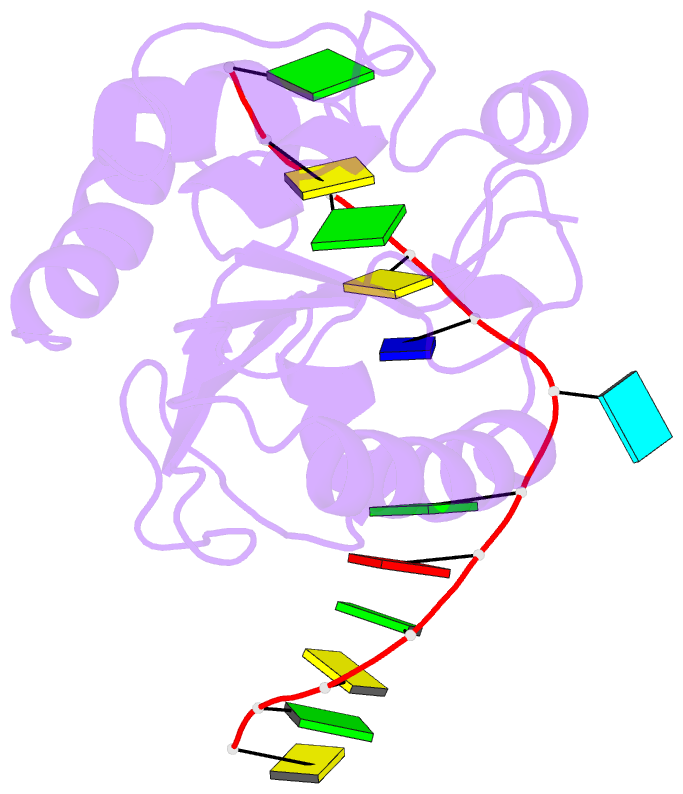
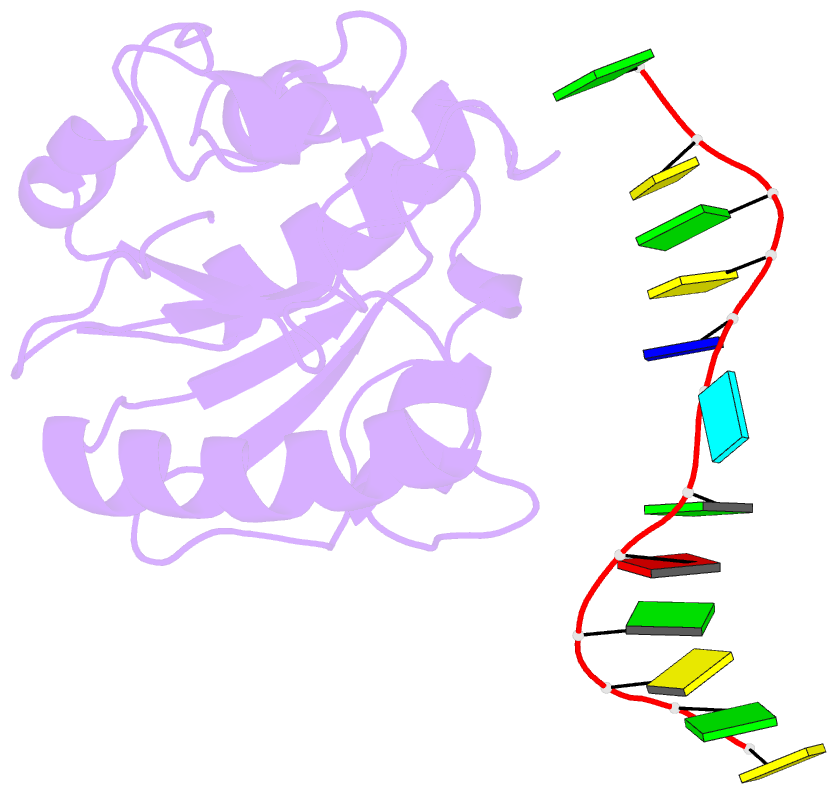
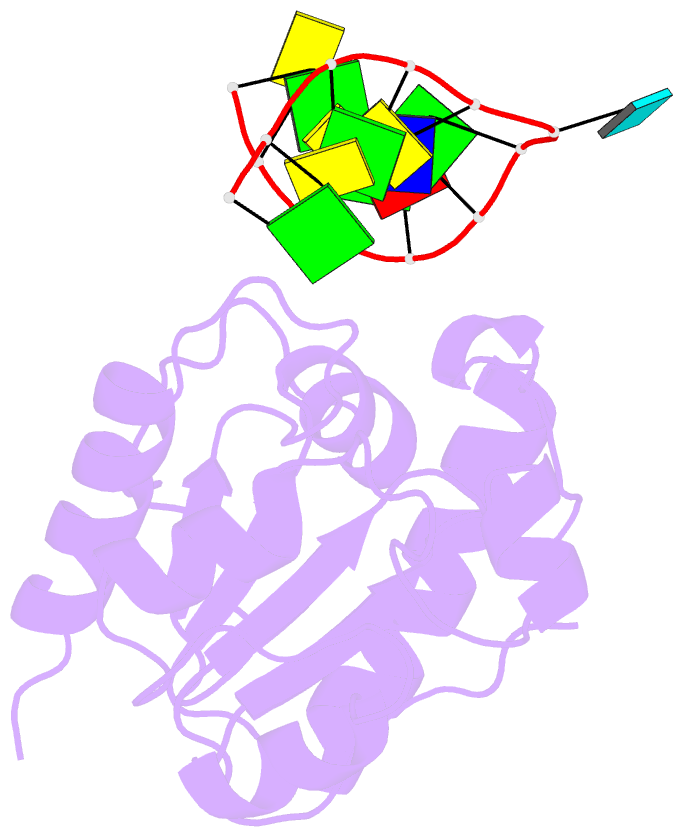
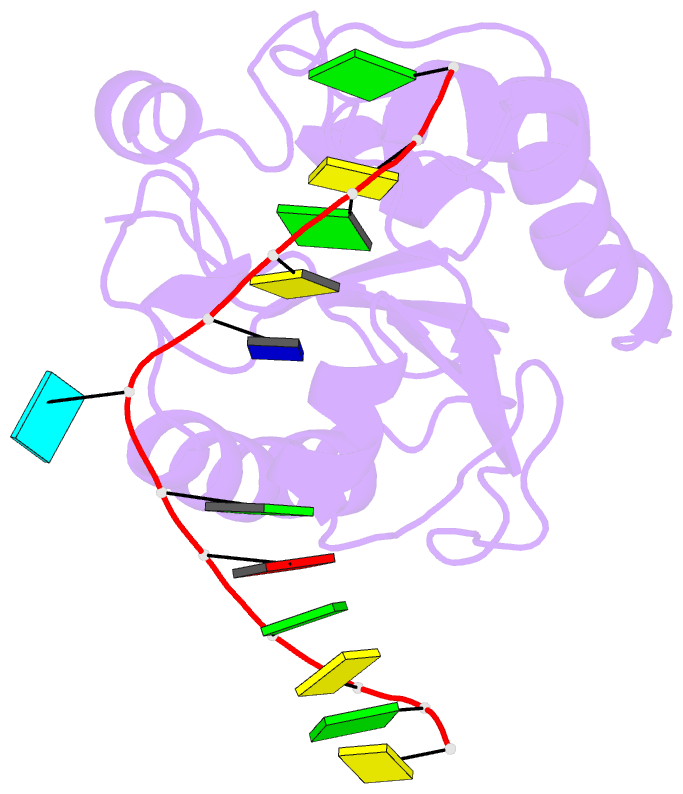
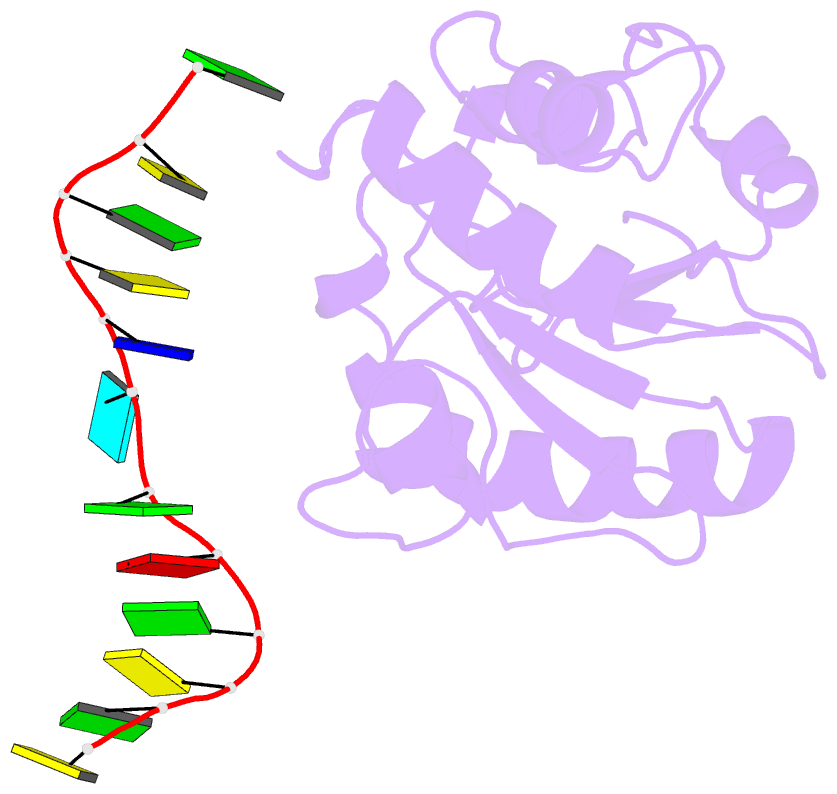
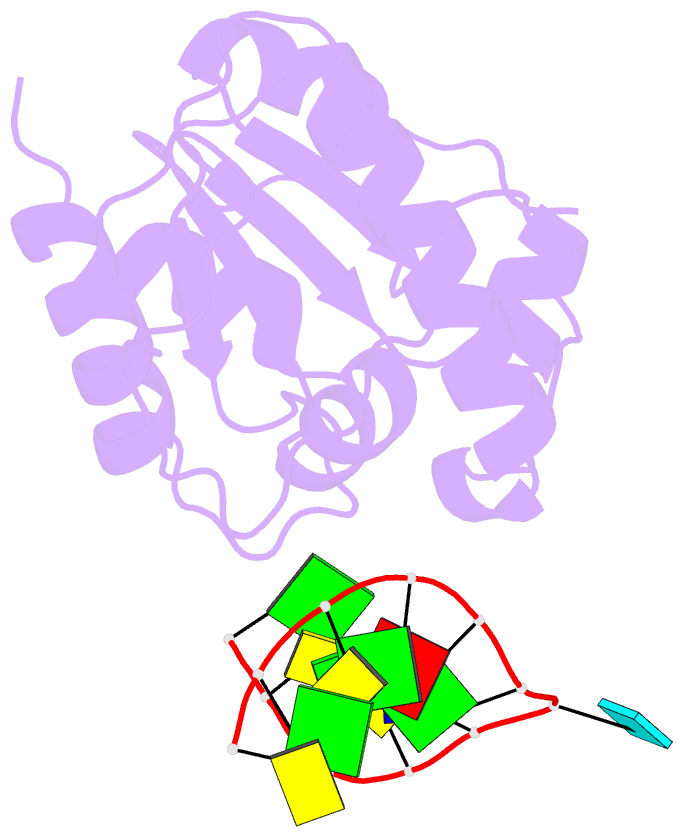
Summary information and primary citation
- PDB-id
- 1mwj; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.85 Å)
- Summary
- Crystal structure of a mug-DNA pseudo substrate complex
- Reference
- Barrett TE, Scharer O, Savva R, Brown T, Jiricny J, Verdine GL, Pearl LH (1999): "Crystal Structure of a thwarted mismatch glycosylase DNA repair complex." Embo J., 18, 6599-6609. doi: 10.1093/emboj/18.23.6599.
- Abstract
- The bacterial mismatch-specific uracil-DNA glycosylase (MUG) and eukaryotic thymine-DNA glycosylase (TDG) enzymes form a homologous family of DNA glycosylases that initiate base-excision repair of G:U/T mismatches. Despite low sequence homology, the MUG/TDG enzymes are structurally related to the uracil-DNA glycosylase enzymes, but have a very different mechanism for substrate recognition. We have now determined the crystal structure of the Escherichia coli MUG enzyme complexed with an oligonucleotide containing a non-hydrolysable deoxyuridine analogue mismatched with guanine, providing the first structure of an intact substrate-nucleotide productively bound to a hydrolytic DNA glycosylase. The structure of this complex explains the preference for G:U over G:T mispairs, and reveals an essentially non-specific pyrimidine-binding pocket that allows MUG/TDG enzymes to excise the alkylated base, 3, N(4)-ethenocytosine. Together with structures for the free enzyme and for an abasic-DNA product complex, the MUG-substrate analogue complex reveals the conformational changes accompanying the catalytic cycle of substrate binding, base excision and product release.