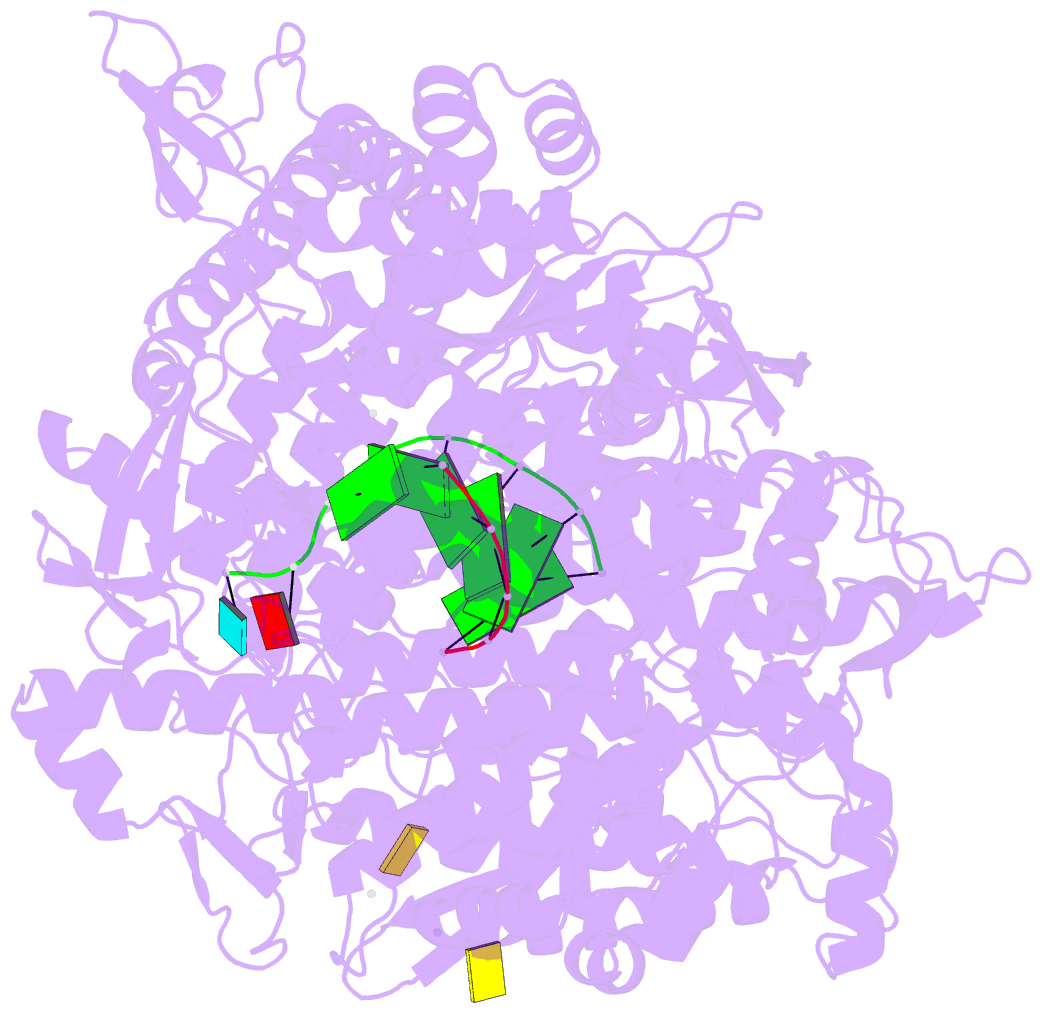
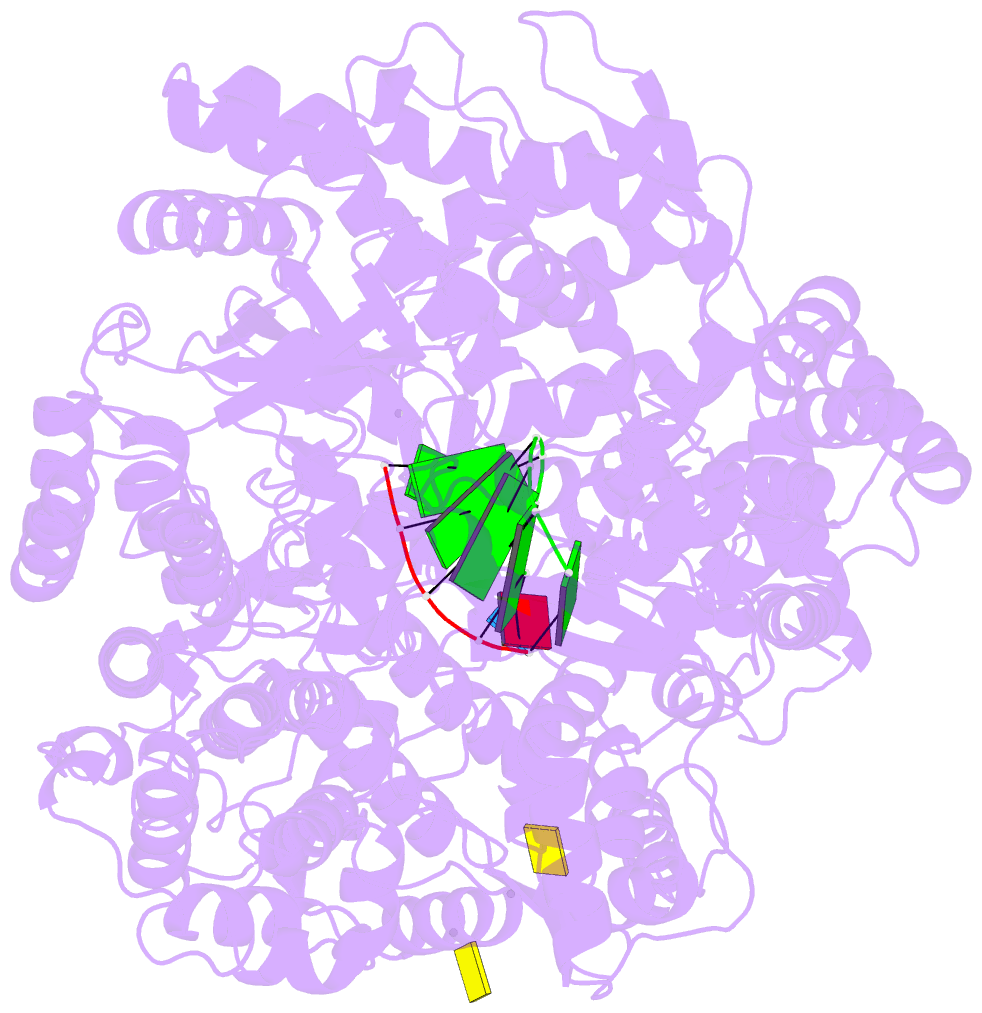
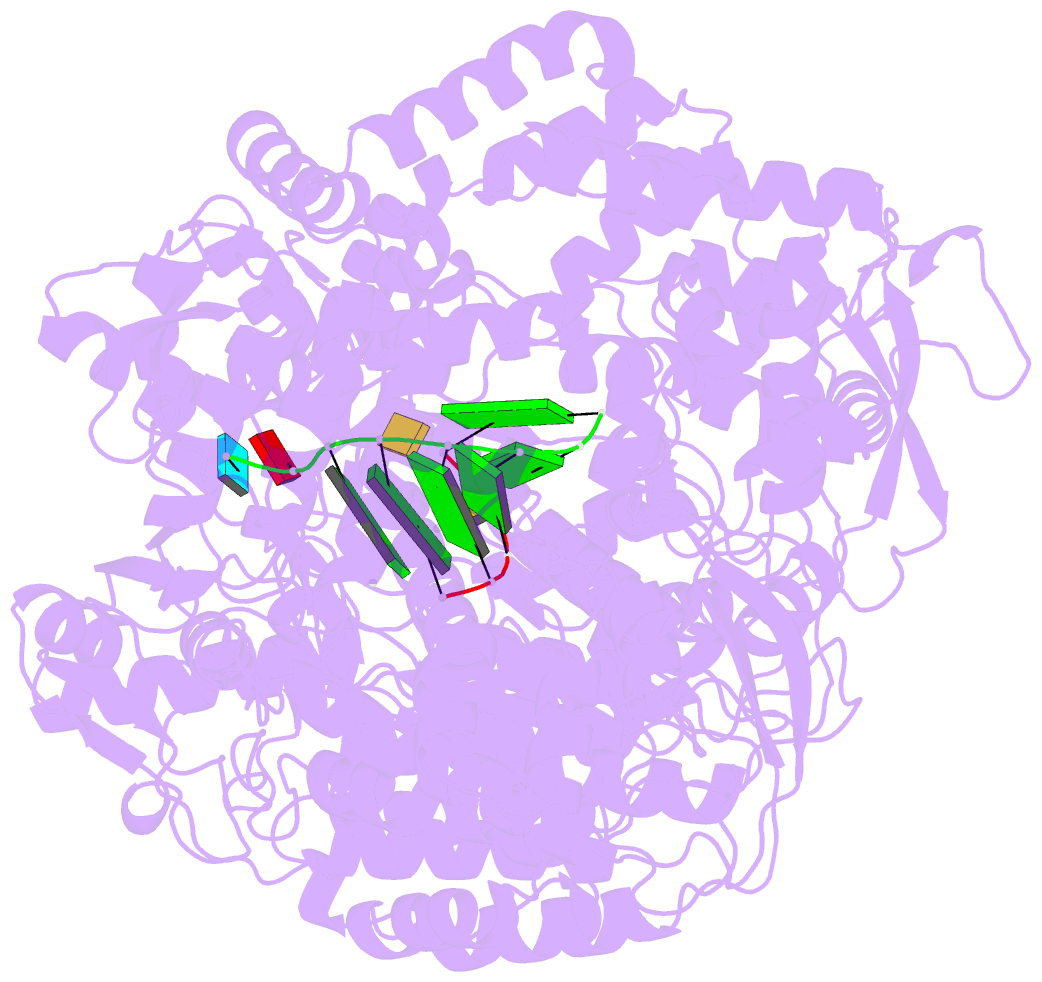
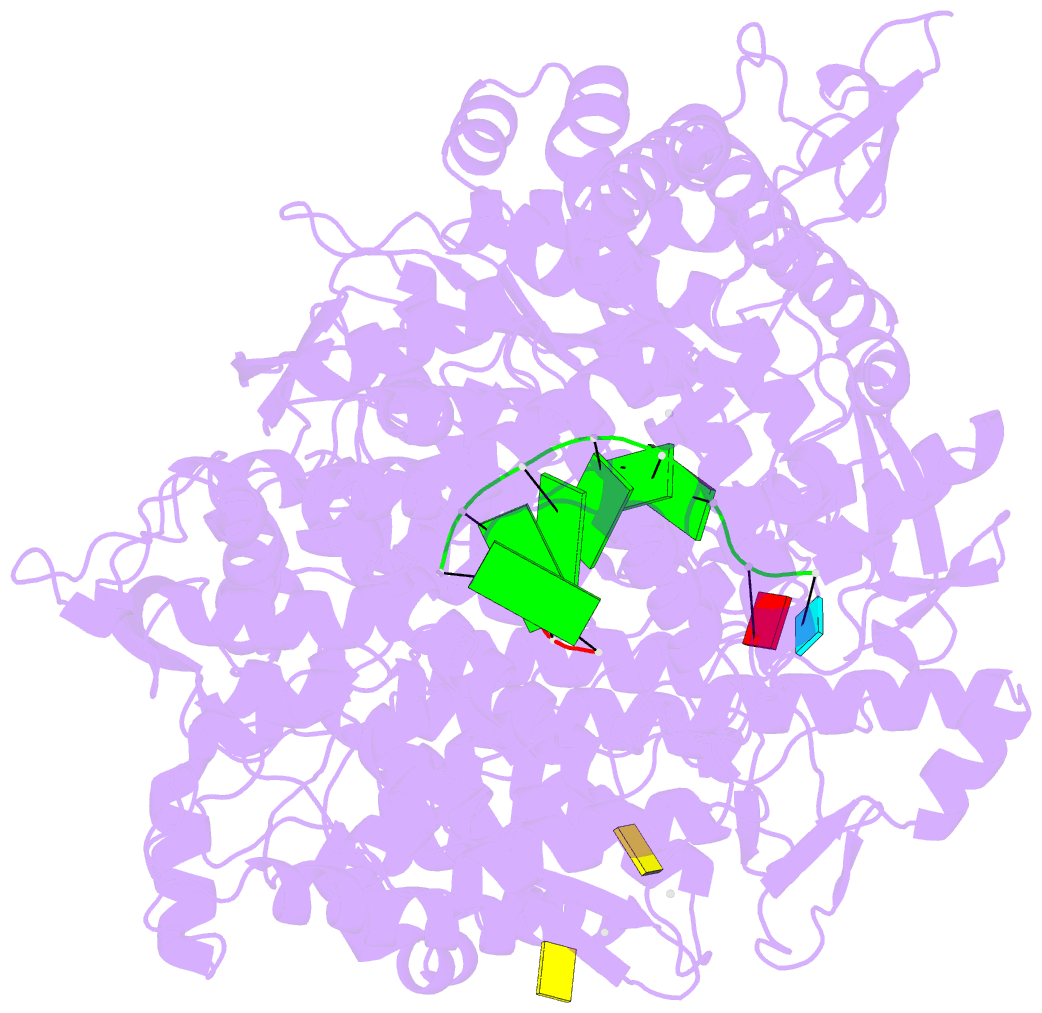
Summary information and primary citation
- PDB-id
- 1n35; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-RNA
- Method
- X-ray (2.5 Å)
- Summary
- Lambda3 elongation complex with four phosphodiester bond formed
- Reference
- Tao Y, Farsetta DL, Nibert ML, Harrison SC (2002): "RNA Synthesis in a Cage--Structural Studies of Reovirus Polymerase [lambda] 3." Cell(Cambridge,Mass.), 111, 733-745. doi: 10.1016/S0092-8674(02)01110-8.
- Abstract
- The reovirus polymerase and those of other dsRNA viruses function within the confines of a protein capsid to transcribe the tightly packed dsRNA genome segments. The crystal structure of the reovirus polymerase, lambda3, determined at 2.5 A resolution, shows a fingers-palm-thumb core, similar to those of other viral polymerases, surrounded by major N- and C-terminal elaborations, which create a cage-like structure, with four channels leading to the catalytic site. This "caged" polymerase has allowed us to visualize the results of several rounds of RNA polymerization directly in the crystals. A 5' cap binding site on the surface of lambda3 suggests a template retention mechanism by which attachment of the 5' end of the plus-sense strand facilitates insertion of the 3' end of the minus-sense strand into the template channel.