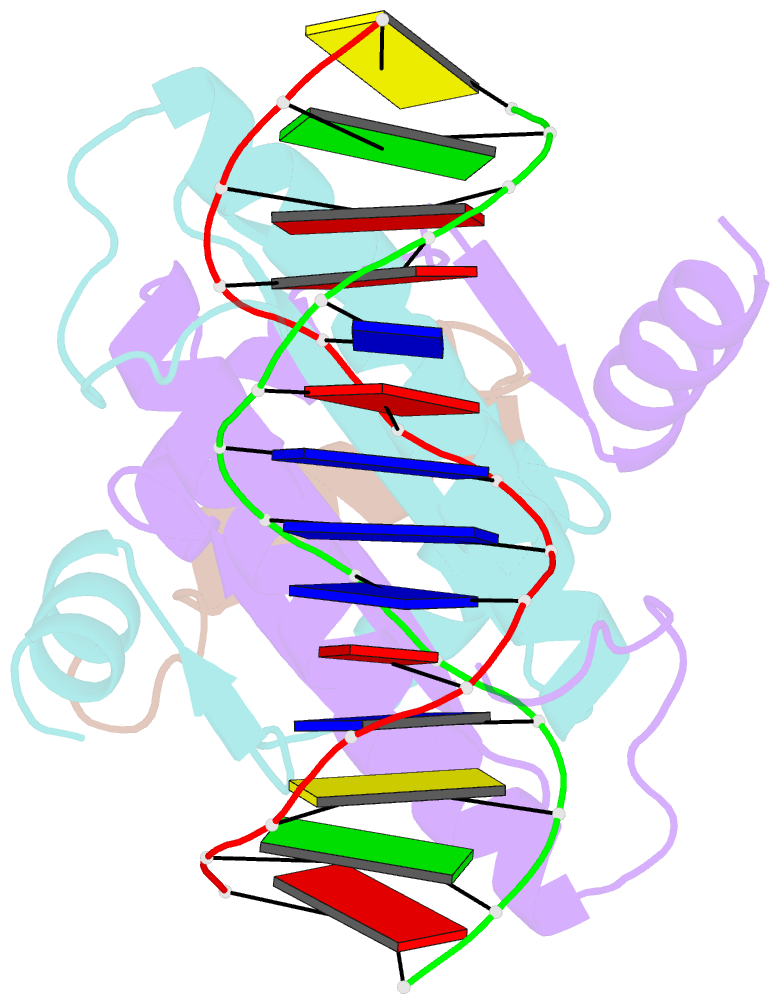
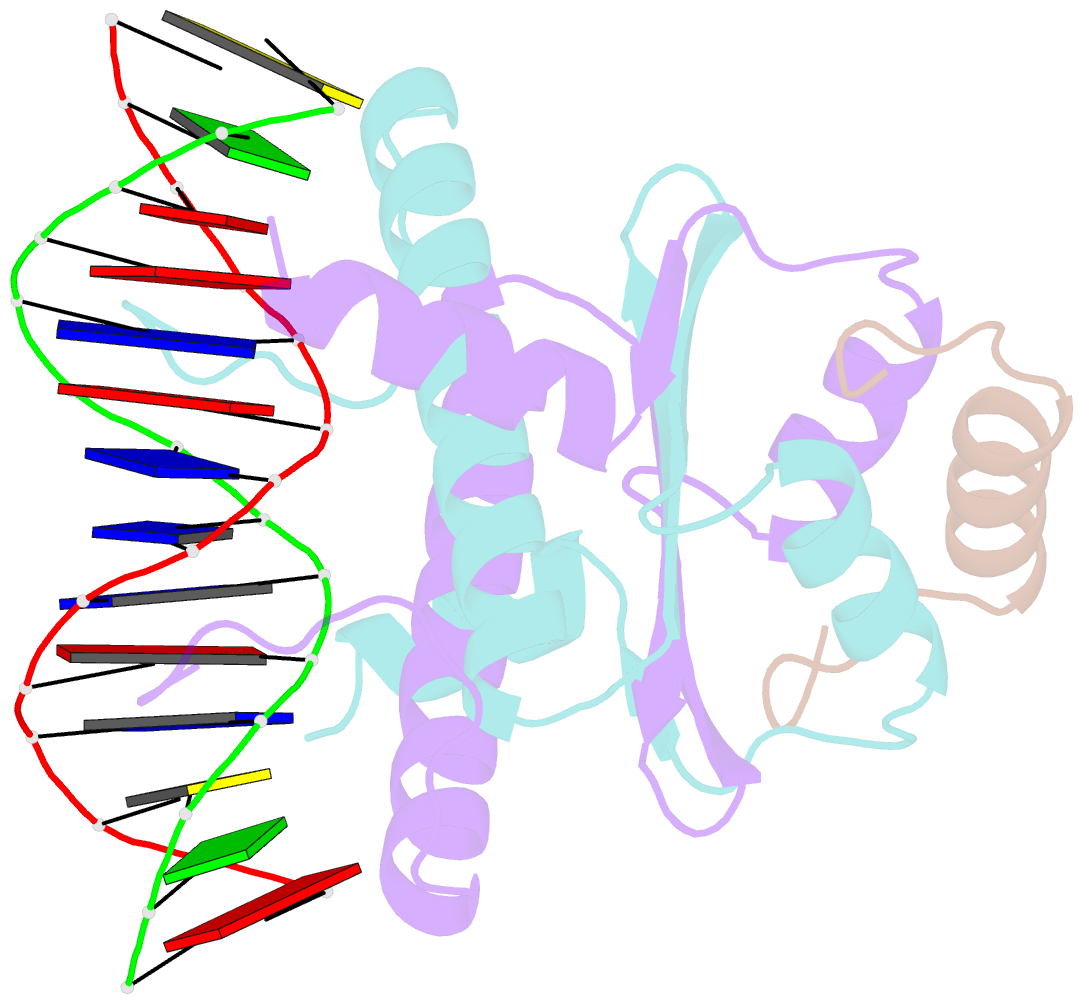
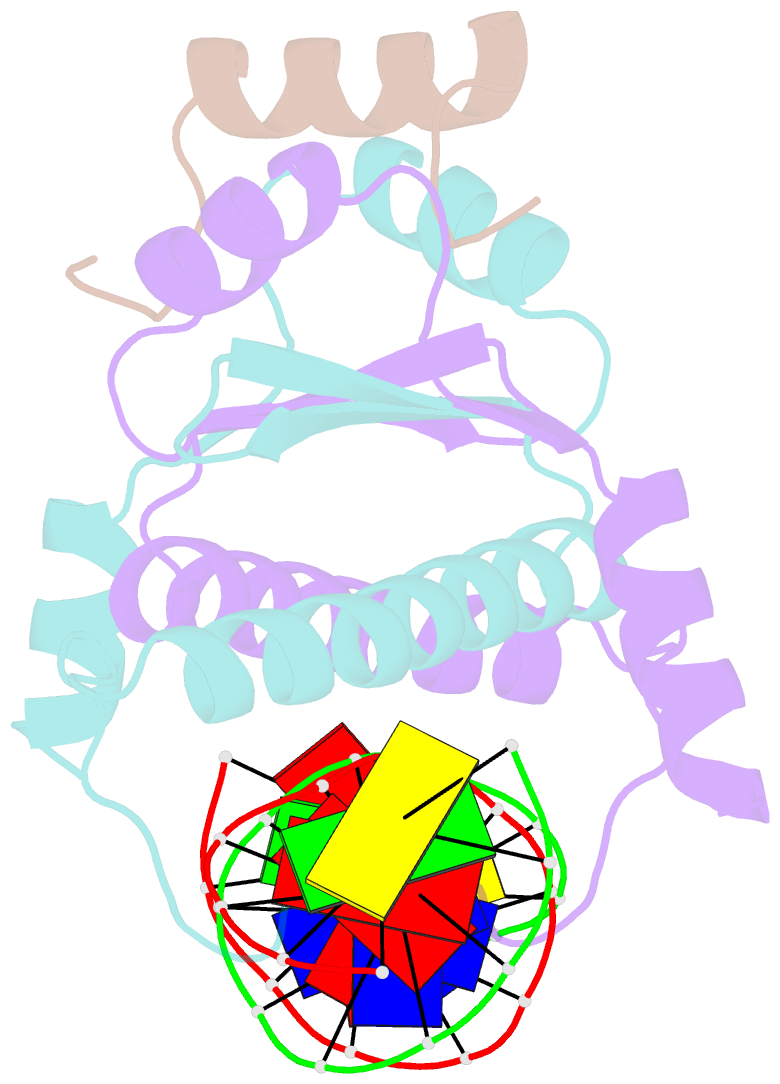
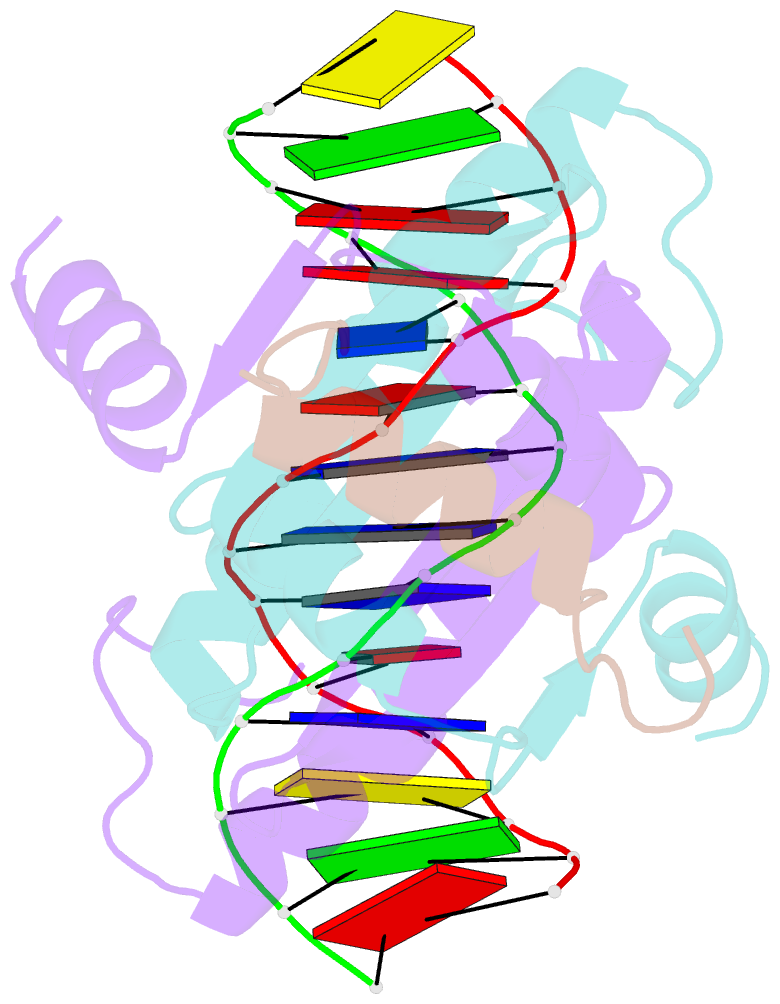
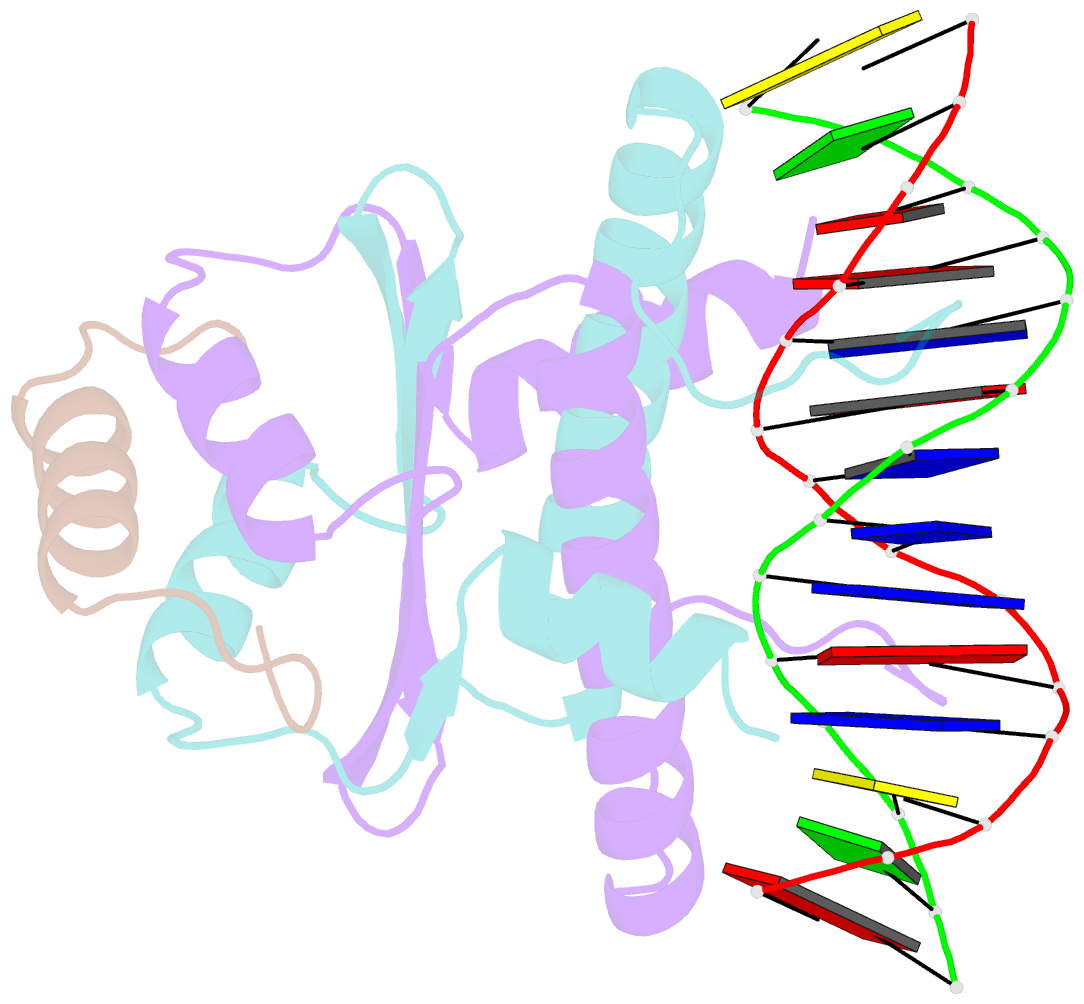
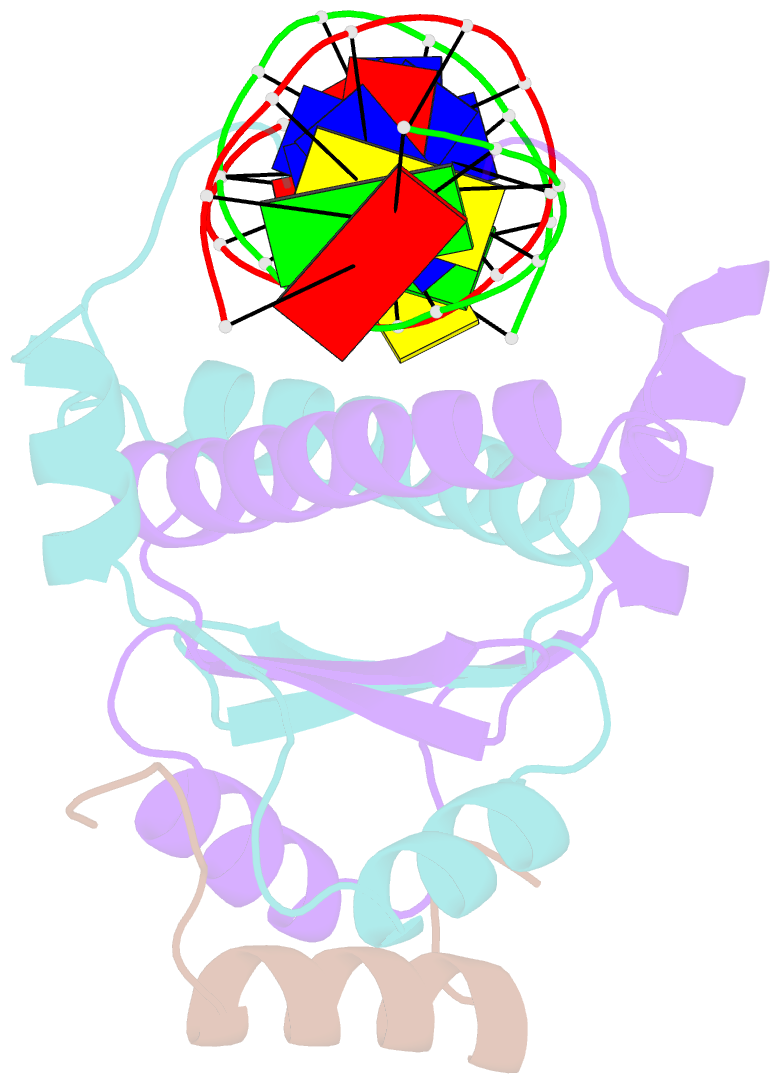
Summary information and primary citation
- PDB-id
- 1n6j; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.2 Å)
- Summary
- Structural basis of sequence-specific recruitment of histone deacetylases by myocyte enhancer factor-2
- Reference
- Han A, Pan F, Stroud JC, Youn HD, Liu JO, Chen L (2003): "Sequence-specific recruitment of transcriptional co-repressor Cabin1 by myocyte enhancer factor-2." Nature, 422, 730-734. doi: 10.1038/nature01555.
- Abstract
- The myocyte enhancer factor-2 (MEF2) family of transcription factors has important roles in the development and function of T cells, neuronal cells and muscle cells. MEF2 is capable of repressing or activating transcription by association with a variety of co-repressors or co-activators in a calcium-dependent manner. Transcriptional repression by MEF2 has attracted particular attention because of its potential role in hypertrophic responses of cardiomyocytes. Several MEF2 co-repressors, such as Cabin1/Cain and class II histone deacetylases (HDACs), have been identified. However, the molecular mechanism of their recruitment to specific promoters by MEF2 remains largely unknown. Here we report a crystal structure of the MADS-box/MEF2S domain of human MEF2B bound to a motif of the transcriptional co-repressor Cabin1 and DNA at 2.2 A resolution. The crystal structure reveals a stably folded MEF2S domain on the surface of the MADS box. Cabin1 adopts an amphipathic alpha-helix to bind a hydrophobic groove on the MEF2S domain, forming a triple-helical interaction. Our studies of the ternary Cabin1/MEF2/DNA complex show a general mechanism by which MEF2 recruits transcriptional co-repressor Cabin1 and class II HDACs to specific DNA sites.