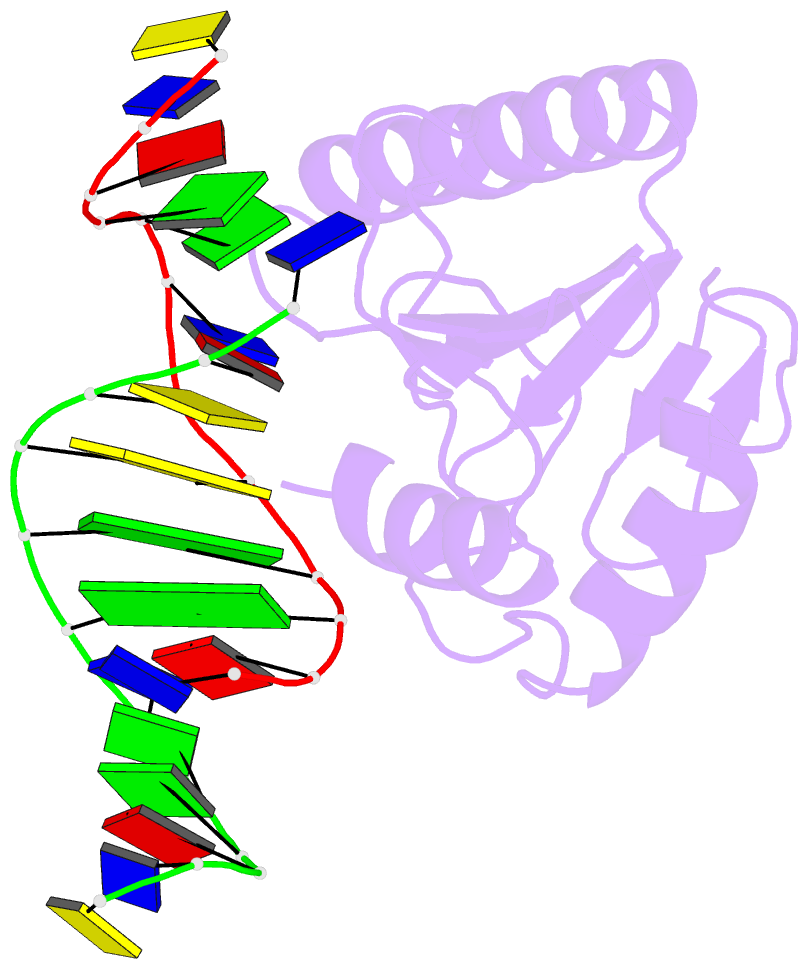
Summary information and primary citation
- PDB-id
- 1odg; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.8 Å)
- Summary
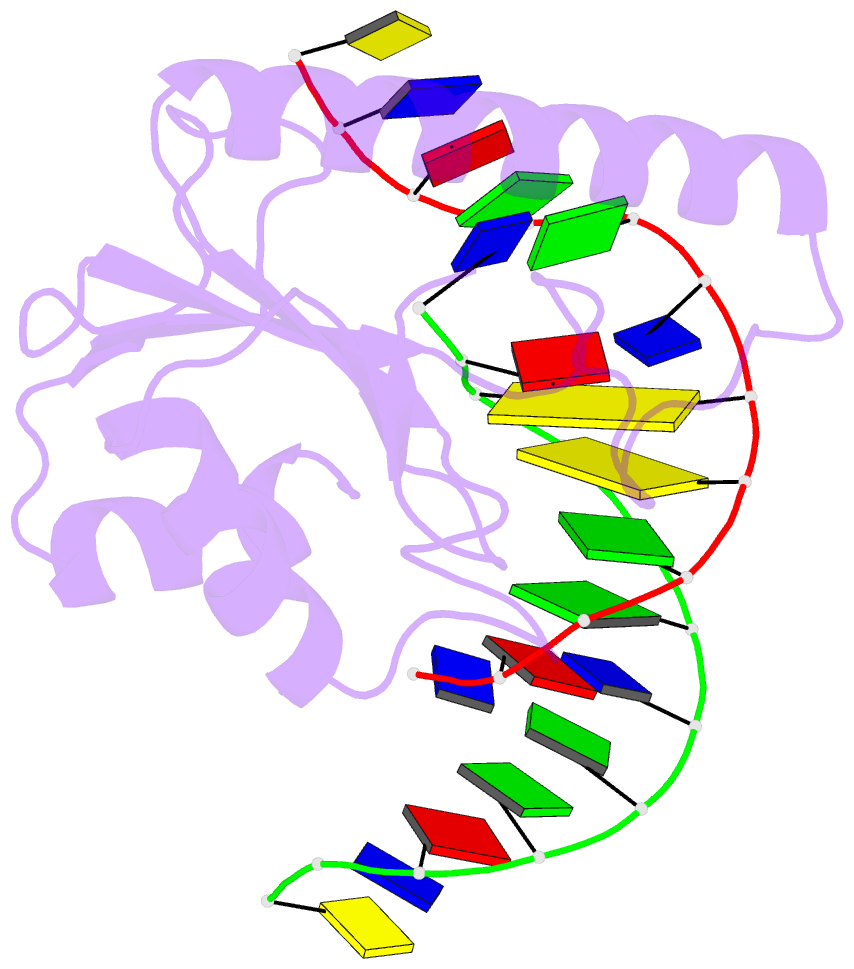
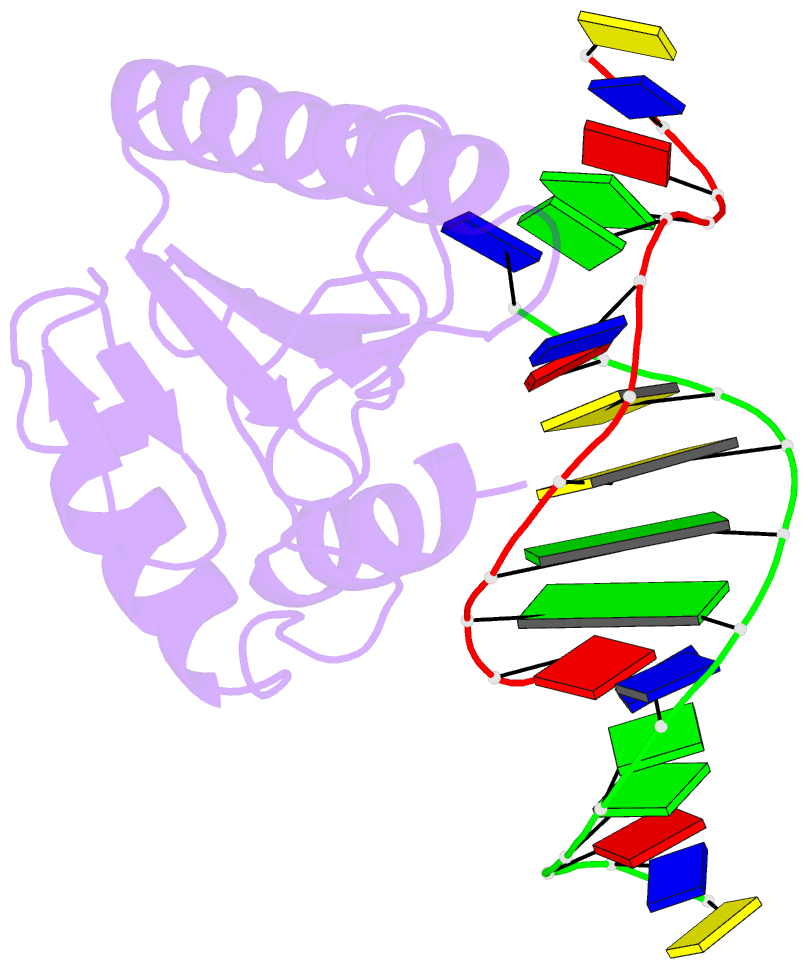
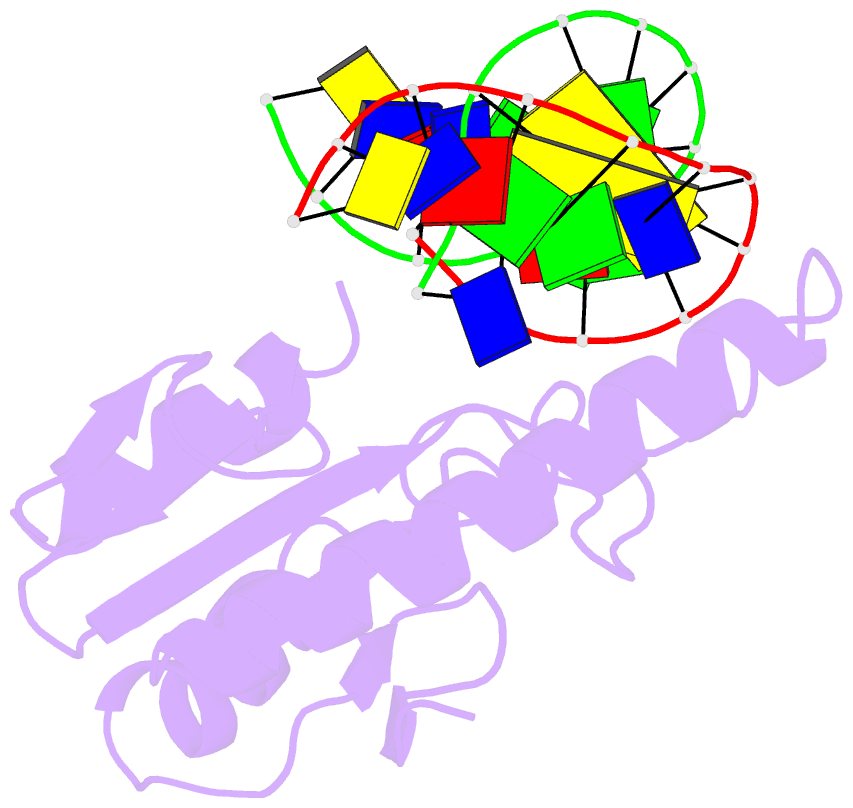
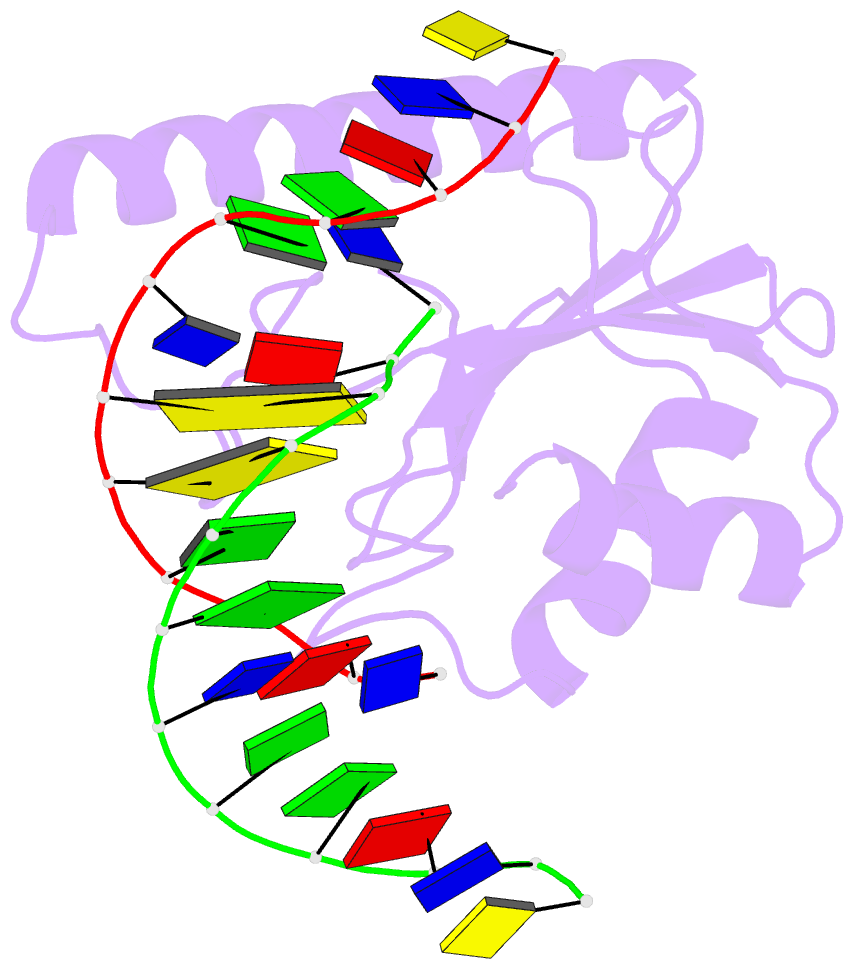
- Very-short-patch DNA repair endonuclease bound to its reaction product site
- Reference
- Bunting KA, Roe SM, Headley A, Brown T, Savva R, Pearl LH (2003): "Crystal Structure of the Escherichia Coli Dcm Very-Short-Patch DNA Repair Endonuclease Bound to its Reaction Product-Site in a DNA Superhelix." Nucleic Acids Res., 31, 1633. doi: 10.1093/NAR/GKG273.
- Abstract
- Very-short-patch repair (Vsr) enzymes occur in a variety of bacteria, where they initiate nucleotide excision repair of G:T mismatches arising by deamination of 5-methyl-cytosines in specific regulatory sequences. We have now determined the structure of the archetypal dcm-Vsr endonuclease from Escherichia coli bound to the cleaved authentic hemi-deaminated/hemi-methylated dcm sequence 5'-C-OH-3' 5'-p-T-p-A-p-G-p-G-3'/3'-G-p-G-p-T-p(Me5)C-p-C formed by self-assembly of a 12mer oligonucleotide into a continuous nicked DNA superhelix. The structure reveals the presence of a Hoogsteen base pair within the deaminated recognition sequence and the substantial distortions of the DNA that accompany Vsr binding to product sites.